What phase of matter is pictured below?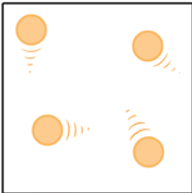
Gas (particles are far apart and moving fast)
What phase(s) are present for line AB?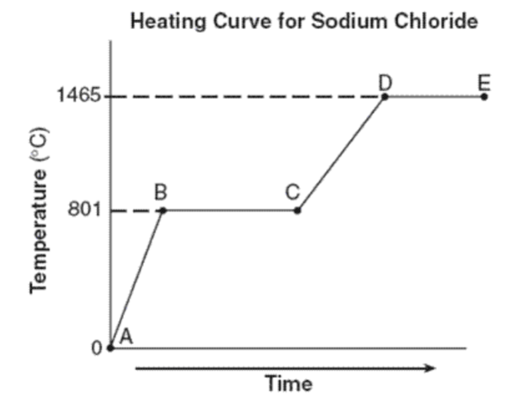
Just solid
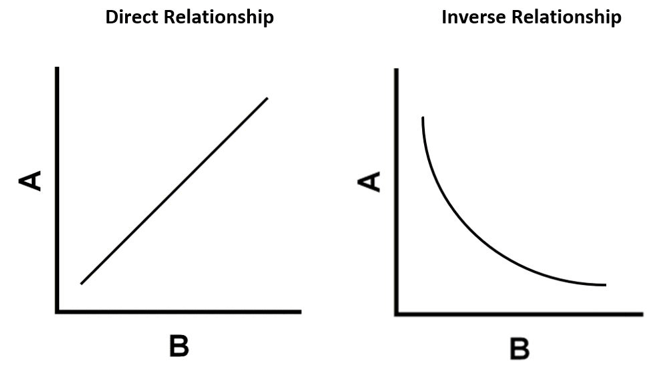
Which graph represents a direct relationship and an inverse relationship?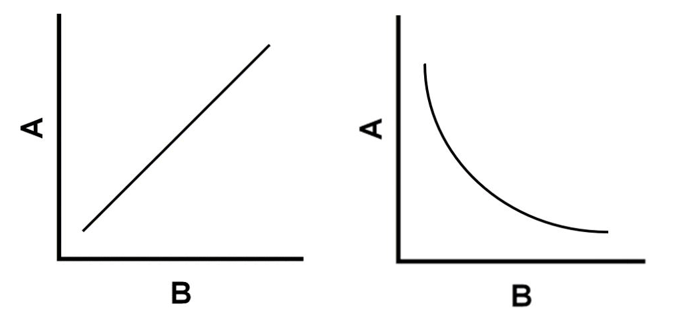
If a gas is at STP, what is the pressure and temperature of that gas?
The gas would be at 1 atm and 273K
What units are necessary for P, V, n, and T in the ideal gas law equation?
P = atm
V = L
n = mol
T = K
What is the phase change called when going from right to left?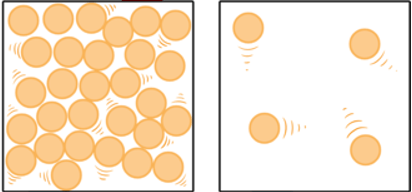
Condensation (gas to liquid)
What is the freezing point of sodium chloride?
What is the melting point of sodium chloride?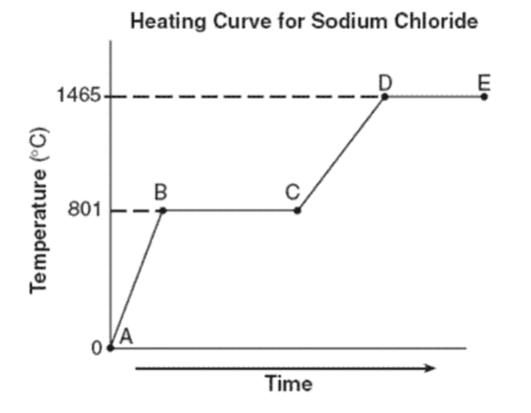
Both freezing point and melting point are the same: 801oC
Which graph represents the relationship between pressure and volume?
Which graph represents the relationship between pressure and temperature?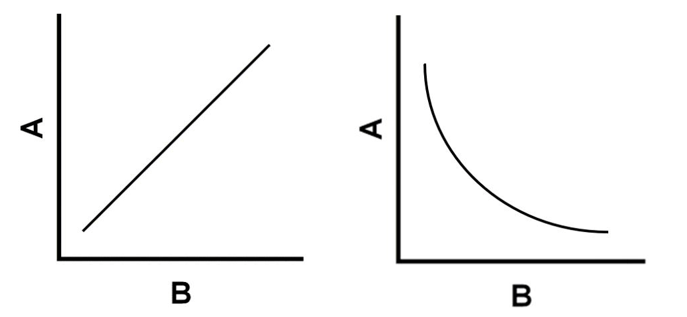
Pressure and volume: inverse relationship
Pressure and temperature: direct relationship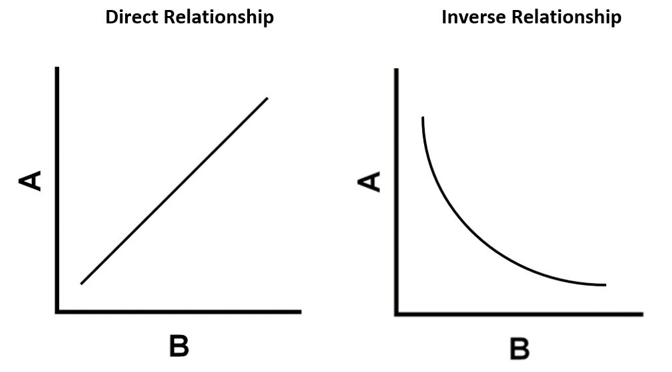
A balloon starts with a pressure of 100 kPa and a volume of 100 mL and is compressed to a pressure of 200 kPa and a volume of 50 mL. The temperature of the balloon stays the same from beginning to the end. What happens to the number of gas particles in the balloon?
The number of gas particles will stay the same even though the pressure and volume are changing.
Convert 880 mmHg to atm.
1.16 atm
What is the phase change called when going left to right? Is this phase change endothermic or exothermic?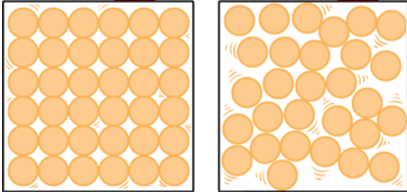
Melting (solid to liquid)
Endothermic (liquid particles have more energy than solid which means that energy was added)
What is happening during BC? Is it a phase change or increasing in temperature?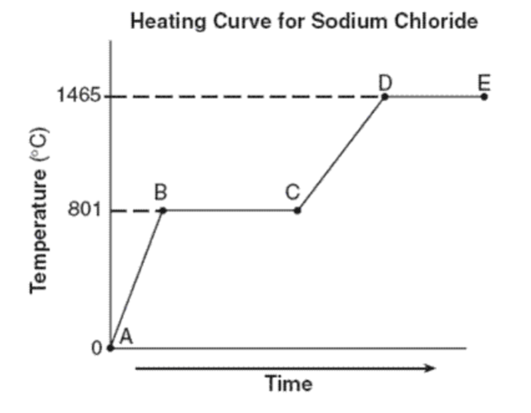
Phase change (phase changes are horizontal lines; increasing temperature are diagonal lines)
If the pressure of a gas increases and temperature remains constant, what should happen to the volume of the gas?
A 25 L sample of gas at 373 K is cooled to 250 K. What is the new volume if the pressure is constant?
16.76 L
Convert 1.2 moles of nitrogen gas to grams of nitrogen gas.
33.62 g N2
What is the phase change called when going from solid to gas? Is this phase change endothermic or exothermic?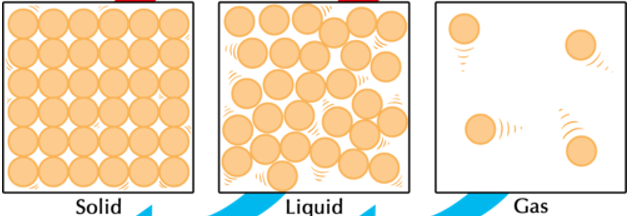
Endothermic (gas particles have more energy than solid which means energy was gained)
During a phase change, why doesn't the temperature change?
Energy is going into breaking the INTERMOLECULAR FORCES which allows the particles to space farther apart and change phases. Energy isn't going into increasing particle movement/temperature.
Increasing the temperature of a gas at a constant volume causes the pressure to increase. Why?
Increasing temperature causes particles to move faster which will cause the particles to collide more. More collisions means more pressure.
A balloon at standard pressure and 20oC is moved to a new location with a pressure of 2.2 atm while maintaining the same volume. What is the new temperature
644.60 K
A 1 L container of oxygen gas has a pressure of 2 atm and is at a temperature of 290 K. How many moles of gas are in the container?
0.084 moles
Compare shape and volume between the 3 phases. (HINT: We used specific words when describing them in class)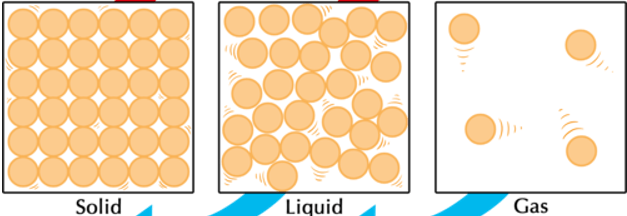
Solid: definite shape and definite volume
Liquid: indefinite shape and definite volume
Gas: indefinite shape and indefinite volume
What phases are present for lines CD? for lines DE?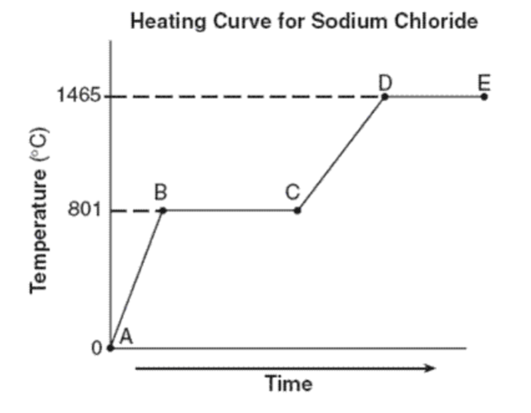
CD: liquid
DE: liquid and gas
If the amount of gas increases inside of a fixed container at a constant temperature, what will happen to the pressure? Explain what is happening to the particles inside the container.
The pressure will increase.
Increasing the amount of gas will cause more collisions between the particles. The particles aren't moving faster, however there are more particles moving in the same amount of space. More collisions means more pressure.
A sample of a gas occupies 10 L at 270 K and 2.1 atm. If it is heated to 340 K and expands to 33 L, what is the pressure of the gas?
0.80 atm
A 30g sample of oxygen gas are present at 2 atm and 24oC. What volume does the oxygen gas occupy?
11.46 L