The image below can be described as a Heterogeneous Mixture or Homogeneous Mixture? 
Homogeneous Mixture
Which separation technique would be best to separate rocks from sand?
Manual Separation (sorting)
The universal solvent is ______.
Water
Which is the most concentrated?
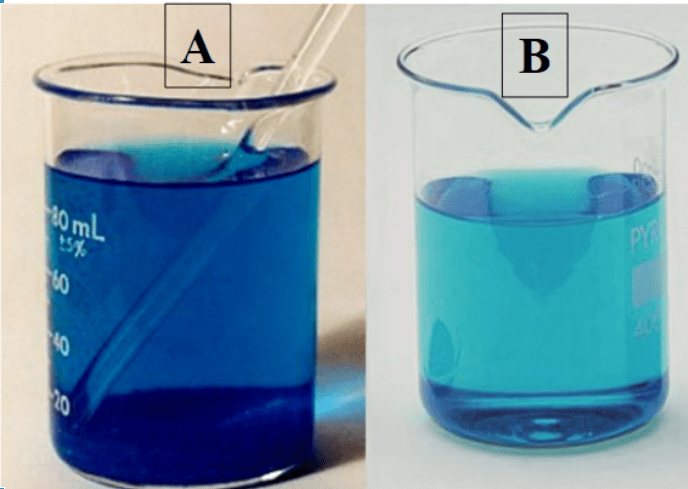
A
Below are ways to increase the solubility of a solid or a gas?
1. Increases temperature
2. Increase surface area
3. Agitate
Solid
Mixtures are separated by physical or chemical methods?
Physical Methods
What separation technique would be the best to separate iron filings from dirt?
Magnetism
18K Gold is 75% gold and the rest is copper and silver. Identify the solvent and solute.
Gold is the Solvent. Copper and Silver are the Solute.
Which is more dilute?
0.10 M or 10.0 M
0.10 M
Which substance is the most soluble at 50°C?
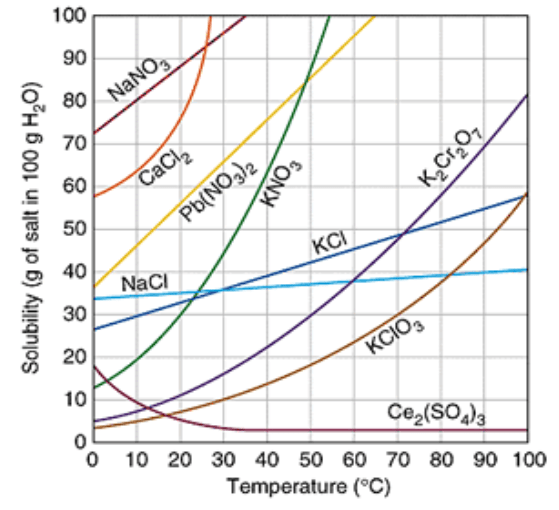
KNO3
The image below shows a mixture of compounds, elements, or both?
Mixture of a Compound & Diatomic Element
Water and ethanol are two liquids. The boiling point of water is 100°C and ethanol is 87°C. What would be the best separation technique to use to separate these two liquids?
The image below represents dissociation or dissolving? Does it represents an electrolyte or non-electrolyte?
Dissociation resulting in an electrolyte that would conduct electricity.
Which is more dilute?
5 L of water or 10 L of water
(in the same amount of solute)
10 L of water
How many grams of KCl will dissolve at 10°C?
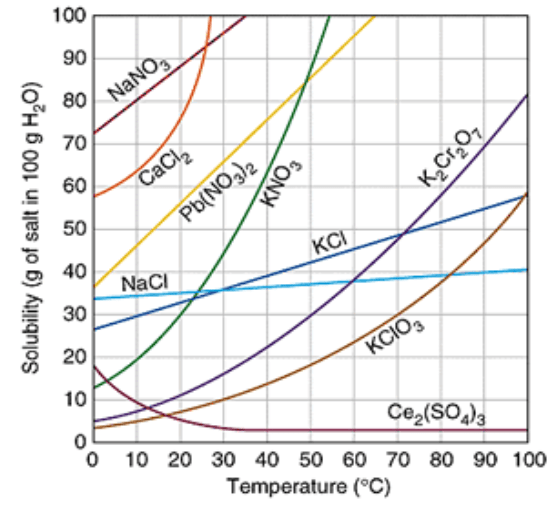
30 g
The warming curve below could represent salt or saltwater?
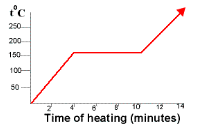
Salt because NaCl is a pure substance resulting in a flat line phase change.
Which separation technique is used to separate the plasma from your blood cells? 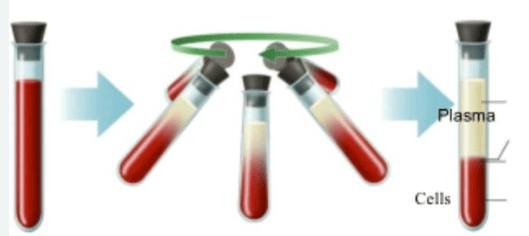
Centrifuging
Chalk does not dissolve in water. These two substances would be considered which of the following?
A) Soluble
B) Insoluble
C) Miscible
D) Immiscible
What is the concentration of 2.0 mol of NaCl dissolved in 10.0 L of water?
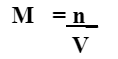
0.20 M
40g of KCl in 100g of water at 80°C would be considered saturated or unsaturated?
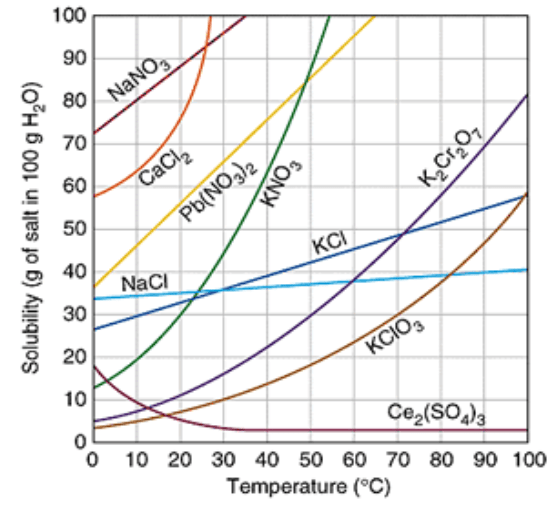
Unsaturated
A homogeneous mixture composed of two or more metals is known as ____________.
an alloy
Test answers were written on a piece of paper in marker. 3 students used a marker on their exams that day. Which separation technique could Mrs. Kline use to identify which marker was used?
Chromatography to separate the ink.
Oil and water do not mix therefore are considered which of the following?
A) Soluble
B) Insoluble
C) Miscible
D) Immiscible
Immiscible - two liquids that do not mix
2.0 L of 10.0 M saltwater are diluted down to a concentration of 5 M. What is the final volume of the solution?
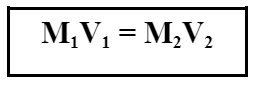
4.0 L
Which substance is a gas?
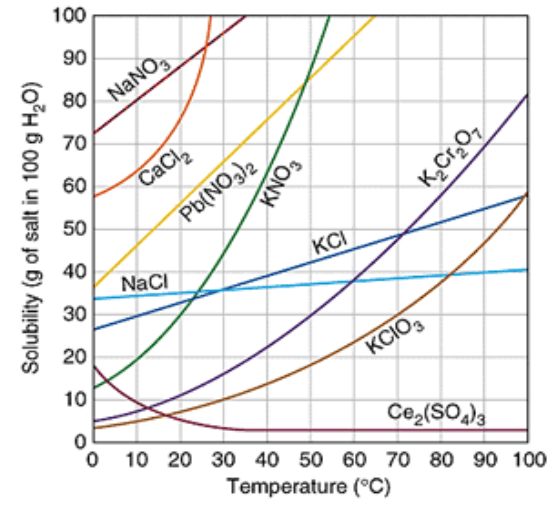
Ce2(SO4)3