What type of bond forms between the following two atoms?:
K and Br
Ionic Bond

Do the following atoms form a polar or non-polar covalent bond?
O and H
Polar
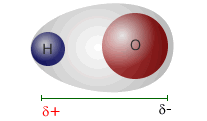
__________ electrons are the electrons involved in bonding.
Valence electrons are the electrons involved in bonding.
How many lone pairs are on the central atom of a trigonal pyramidal molecule?
One!
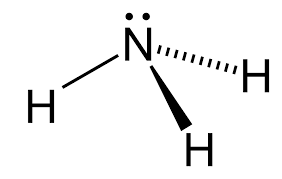
What type of bond forms between the following two atoms:
O and H
Covalent Bond
Do the following atoms form a polar or non-polar covalent bond?
O and O
Non-Polar
 O2
O2
Atoms follow this rule while achieving noble-gas electron configurations.
The octet rule
True or False
VSEPR stands for 'Valence Shell Electron Pair Repulsion"
TRUE
What type of bond forms between the following two atoms:
Cl and Cl
Covalent Bond

Do the following atoms form a polar or non-polar covalent bond?
C and Cl
Polar
Metals _______ electrons to achieve noble-gas electron configurations.
Metals lose (or "give up") electrons to achieve noble-gas electron configurations.
TRUE or FALSE
Electron pairs will get as far away as possible from one another while surrounding an atom.
TRUE
The electron pairs REPEL each other.
What type of bond do the following atoms form:
C and O
Covalent Bond

Do the following atoms form a polar or non-polar covalent bond?
N and N
Non-polar
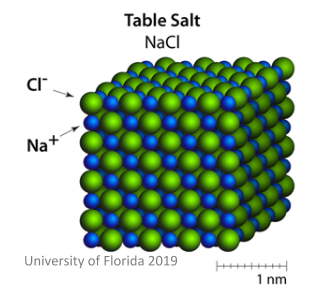 Ionic salts are made from positive cations and negative __________, ionically bonded together.
Ionic salts are made from positive cations and negative __________, ionically bonded together.
Ionic salts are made from cations and negative anions, ionically bonded together.
How many bonding pairs of electrons does the following molecule have? (PCl3)

3

The attraction between a positive metal ion and the electrons surrounding it is a(n)________________ bond.
The attraction between a positive metal ion and the electrons surrounding it is a(n) __METALLIC bond.

Do the following atoms form a polar or non-polar covalent bond?
Na and S
Neither! This is an ionic bond


In the molecule Cl2 , the two shared electrons give each chlorine atom ___ electrons in the outer energy level.
In the molecule Cl2 , the two shared electrons give each chlorine atom 8 electrons in the outer energy level.

What is the molecular geometry of the NH3 molecule?

Trigonal Pyriamidal
