The measure of the average kinetic energy of the atoms or molecules in the system
Solid to liquid
What is melting?
The Delta H is negative and it is an exothermic reaction
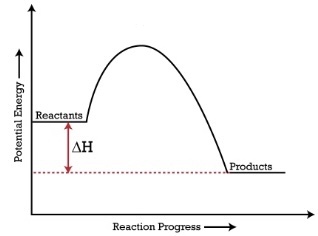 The graph has a lower energy than the reaction.
The graph has a lower energy than the reaction.
What is the definition?
"if a reaction can be broken into a series of steps, the sum of the ΔH of those steps are equal to the ΔH of the reaction."
Which of the following is defined by the measure of the average kinetic energy?
A) Heat
B) Temperature
C) Potential Energy
D) Velocity
B)
The flow of energy between two substances at different temperatures.
What is Heat?
Liquid to Solid
What is freezing?
The Delta H is positive and it is an endothermic reaction.
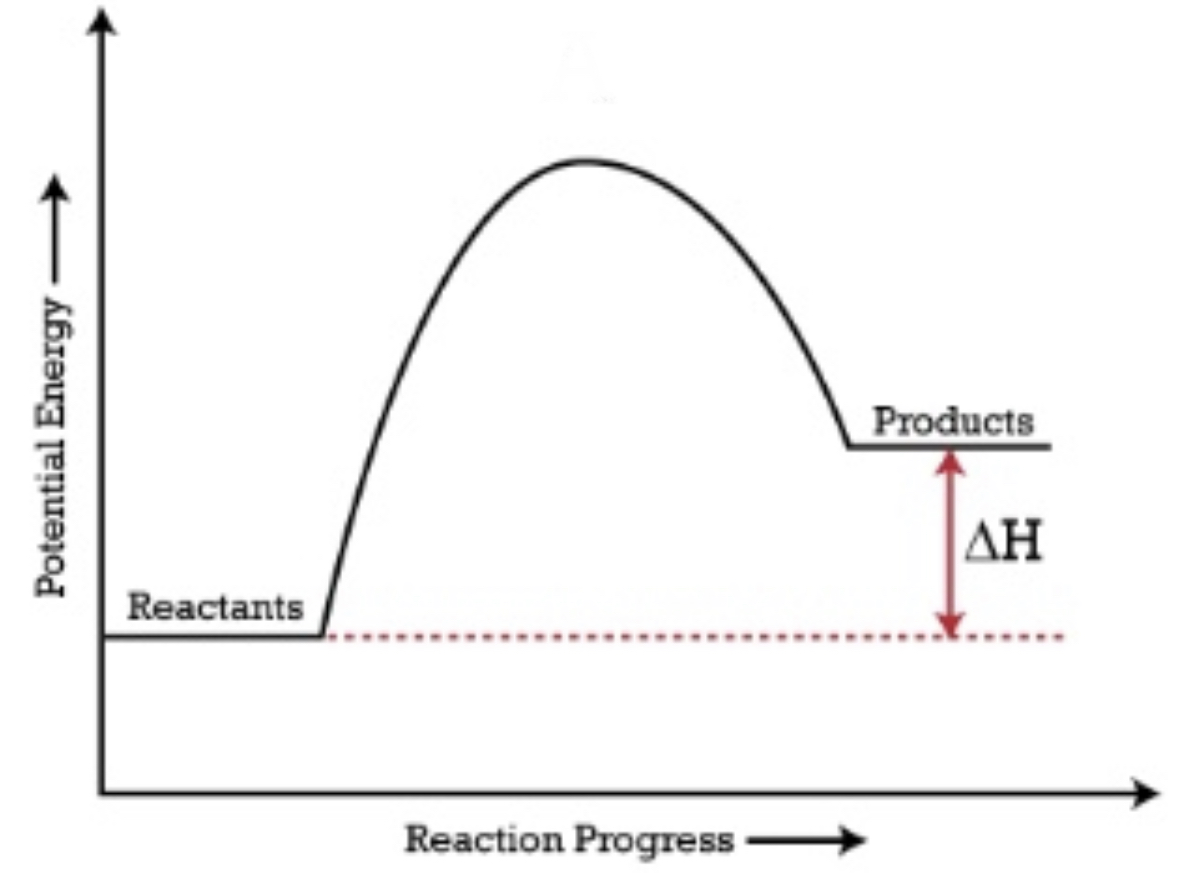 The reactants have a lower energy than the products.
The reactants have a lower energy than the products.
What is the change in enthalpy for the following goal equation?
CS2 + 2 H2O --> CO2 + 2 H2S
Given the following information:
H2S + 3/2 O2 --> H2O + SO2 ΔH = -563 kJ
CS2 + 3 O2 --> CO2 + 2 SO2 ΔH = -1075 kJ
51 kJ :D
In a bond energy calculation experiment, a student finds that the energy required to break bonds is greater than the energy released when new bonds form. What does this indicate about the overall reaction?
A)Isothermal
B)Adiabatic
C)Exothermic
D)Endothermic
Endothermic
This States that "Energy cannot be created or destroyed and can only transfer or change forms."
What is the first law of thermodynamics?
Gas to Liquid
What is condensation?
This only changes the activation energy
 Adding a Catalyst
Adding a Catalyst
What is the change in enthalpy for this goal equation?
NO + O --> NO2
Given the following information:
2 O3 --> 3 O2 ∆H = -427 kJ
O2 --> 2 O ∆H = +495 kJ
NO + O3 --> NO2 + O2 ∆H = -199 kJ
-233 kJ
A student observes that a reaction mixture absorbs heat from the surroundings but does not increase in temperature. What is most likely occurring in the mixture?
A) Temperature Increase
B) Phase Change
C) Cooling process
D) Catalytic reaction
B)
When energy is transferred from the system to the surroundings.
What is Exothermic?
Solid to gas
What is Sublimation
In a chemical reaction, the difference between the potential energy of the products and the potential energy of the reactants to the
Heat of reaction
What is the change in enthalpy for the following goal equation?
H2SO4(l) → SO3(g) + H2O(g)
Using the following information:
| H2S(g) + 2O2(g) → H2SO4(l) ΔH = -235.5 kJ
| H2S(g) + 2O2(g) → SO3(g) + H2O(l) ΔH = -207 kJ
| H2O(l) → H2O(g) ΔH = 44 kJ |
72 kJ
A student investigating Hess's Law used three reactions to determine the enthalpy change of a target reaction. They accurately measured the ΔH for each step but concluded that the overall ΔH for the target reaction was positive, contrary to expected results. What mistake did the student make?
A) Incorrectly summed the ΔH values of the steps
B) Neglected to consider the state of the reactants and products.
C) Used incorrect coefficients for one of the reactions.
D) Failed to reverse the ΔH value of a flipped reaction
A)
When reactions occur when a system absorbs the energy from the surroundings.
What is Endothermic
Gas to Solid
What is deposition?
Which of the following could this energy diagram refer to?
A) N2(g) + O2(g) → 2NO(g)
B) 2C(s) + H2(g) → C2H2(g)
C) H2(g) +I2(g) → 2HI(g)
D) C(s) +O2(g) → CO2(g)
D)
What is the change in enthalpy for the following reaction?
1/2H2(g) + 1/2Cl2(g) → HCl(g)
Given the following standard enthalpies:
COCl2(g) + H2O(l) → CH2Cl2(l) + O2(g) ΔH = 47.5 kJ
2HCl(g) + 1/2O2(g) → H2O(l) + Cl2(g) ΔH = 105 kJ
CH2Cl2(l) + H2(g) + 3/2O2(g) → COCl2(g) + 2H 2O(l) ΔH = -402.5 kJ|
-230 kJ
A researcher conducted an experiment to determine the enthalpy change of a reaction between sodium hydroxide and hydrochloric acid. They mixed equal volumes of 1M NaOH and 1M HCl in a calorimeter and measured the temperature change. They concluded the reaction was endothermic because the solution's temperature decreased. What mistake did the researcher make?
Select the matching term
A) Failed to account for the calorimeter's heat capacity.
B) Used incorrect concentrations, affecting the ΔH calculation.
C) Misinterpreted the temperature change; the reaction is exothermic.
D) Incorrectly measured the temperature change of the reaction.
C)
