This bond forms when one atom gives up at least one electron to another atom.
What is an Ionic Bond?
Name the following functional group?

What is ester?
What is isopentane or 2-methyl-butane?
Draw H20 and name the shape.

Bent
Name HBr
Hydrogen bromide
This bond is formed when two atoms share three electrons.
What is a triple covalent bond?
:max_bytes(150000):strip_icc()/Hydroxyl-1--57ea8c585f9b586c35d47b8a.png)
What is alcohol?

What is 1-Pentene
Draw CH3Cl and give individual bond polarity.

C-Cl → Polar Covalent
C-H → Polar Covalent
Name Li3P
tri-lithium phosphide
This bond is formed by the sharing of one electron between atoms.
What is a covalent bond?

What is carboxylic acid?
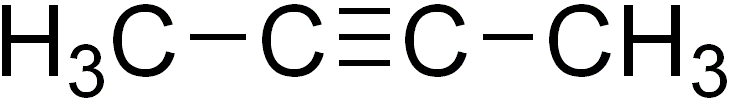
2-Butyne
Draw CH3NH2 and give molecular polarity.
Polar
Name MnS2
magnesium disulfide
This bond is formed from the attraction between mobile electrons and fixed, positively charged metallic atoms.
What is a Metallic Bond?
:max_bytes(150000):strip_icc()/Ether-57ea9db93df78c690fa4e9e4.png)
What is ether?

2-ethyl-3,5-dimethyl-hexane
Draw CO(NH2)2 and give molecular polarity.
2CO.png)
Polar
Name CFl4
Carbon tetrafluoride
This bond is formed by the sharing of two electrons between atoms.
What is a double covalent bond?
:max_bytes(150000):strip_icc()/phenyl-59399b103df78c537ba2605e.jpg)
What is a benzene ring?
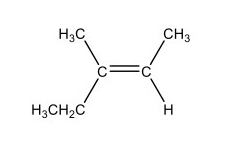
What is cis-3-methyl-2-pentene
Draw C6H6 (Benzene)
Name FePO4
iron(II) phosphate