Which form of matter has a firm and stable shape and is not fluid?
A SOLID
True or false?
The particles in a solid are constantly in motion
TRUE due to the Kinetic Molecular Theory which states that particles are always in motion
Intermolecular forces are forces of attraction ________ molecules
Intermolecular forces are forces of attraction between molecules
Which vocabulary word best describes this picture?
SOLID (or KMT)
Which form of matter conforms to its container and keeps its volume?
A LIQUID
True or false?
Gas particles do not move when they are inside a container
FALSE because particles are always in motion according to the Kinetic Molecular Theory
A _________ will keep its shape and volume.
A solid will keep its shape and volume.
Which vocabulary word best describes this picture?
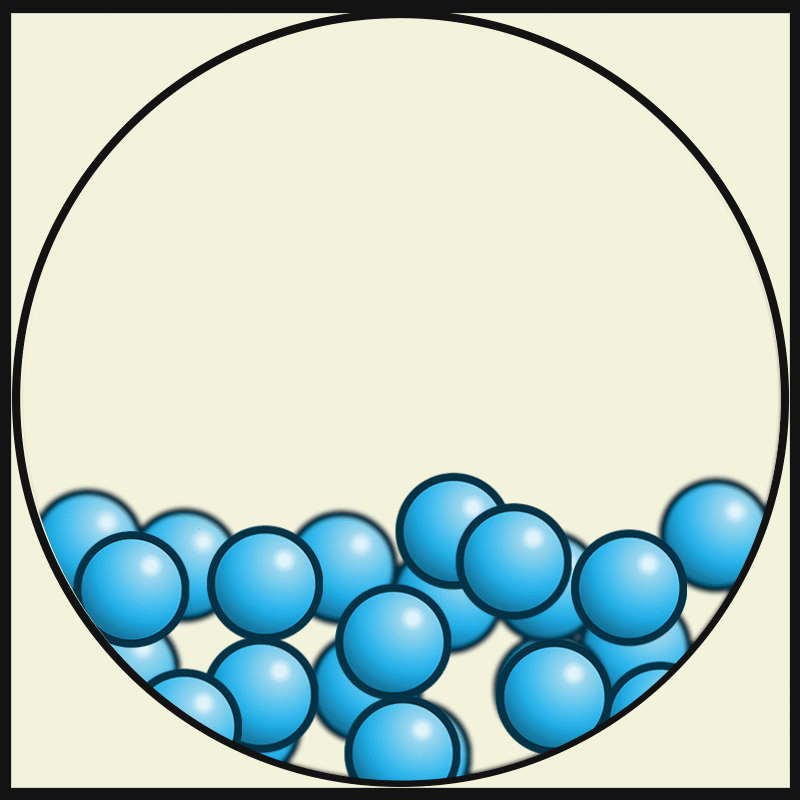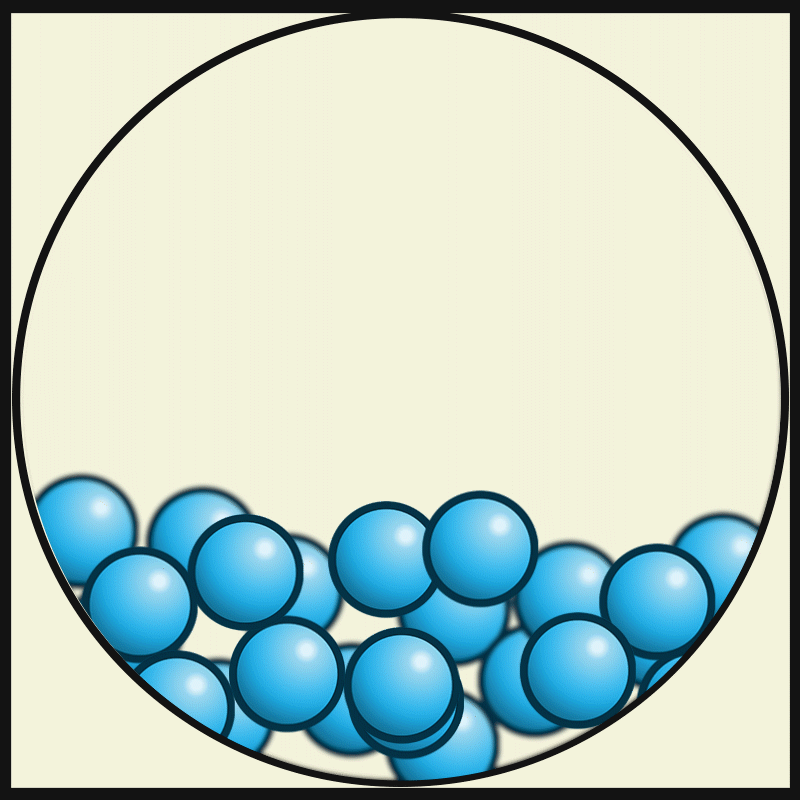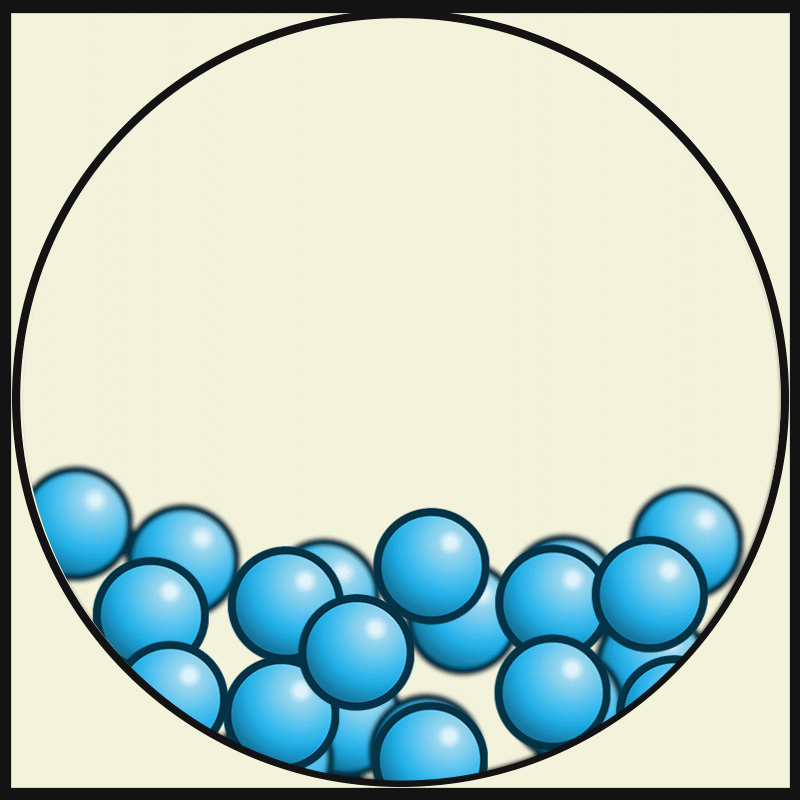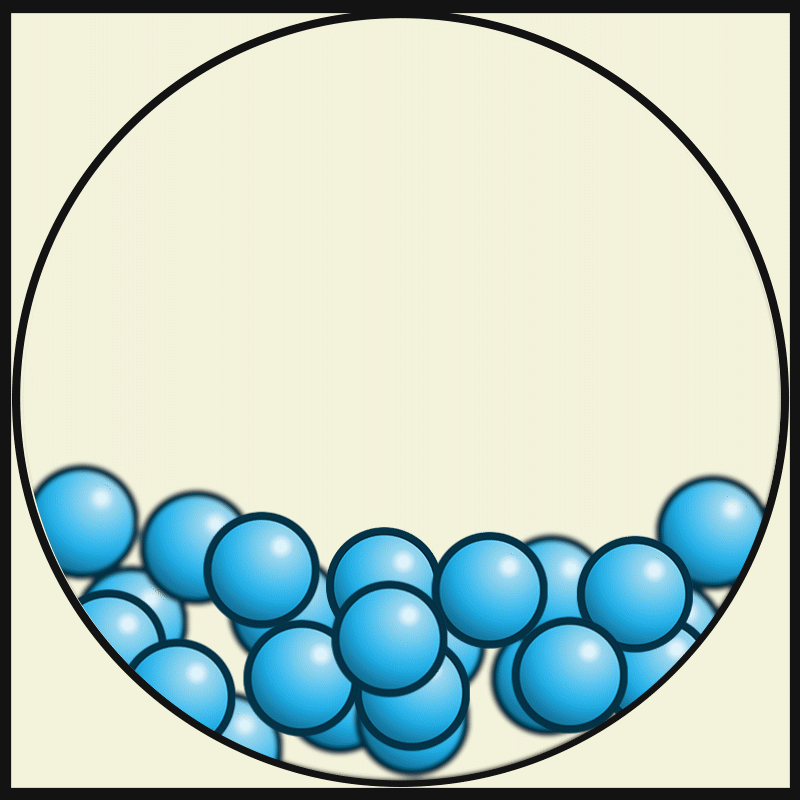
LIQUID
Which form of matter has no definitely shape or volume and will expand to whatever its container is?
A GAS
True or false?
Hydrogen bonding is the strongest type of intermolecular force

TRUE because of the electronegativity difference (Ex. In water, oxygen has a strong pull for electrons, hydrogen has a weak pull for electrons)
The ________________ is a theory that states that all particles are always moving.
The kinetic molecular theory is a theory that states that all particles are always moving.
Which vocabulary word best describes this image? 
Kinetic Molecular Theory or Gas
What is the correct vocabulary word for this definition?
The forces of attraction BETWEEN molecules such as London dispersion forces, dipole-dipole bonding, and hydrogen bonding
Intermolecular forces or IMFs
True or false?
When a solid melts into a liquid, the particles in a liquid move faster than the particles as a solid
TRUE because particles in a liquid can flow but particles in a solid just vibrate
Strong _________________ will hold particles tightly together, affecting how fast or slow the substance will evaporate.
Strong intermolecular forces will hold particles tightly together, affecting how fast or slow the substance will evaporate.
Which vocabulary word best describes this picture?
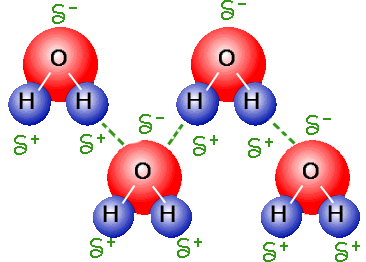
Intermolecular forces or hydrogen bonding
What is the correct vocabulary word for this definition?
An argument that states that particles are constantly moving
Kinetic Molecular Theory or KMT
True or false?
If a substance's intermolecular forces are strong, the substance is more likely to evaporate
(Hint: Think back to the evaporation demo)
FALSE. If there is a strong bond, the strong bond will keep the particles in place and will not allow the particles to move too freely too quickly
Because water molecules have a slightly negative part and a slightly positive part, it is considered to have ___________.

Because water molecules have a slightly negative part and a slightly positive part, it is considered to have polarity.

Which vocabulary word best describes this picture?

Surface tension