Elements in this energy level/block have more than one possible oxidation number or charge
What is D block or transitional metals?
TRUE OR FALSE A chemical bond results from the attraction between the nucleus of one atom and the electrons of another atom.
What is True?
Name this compound FeS
What is Iron (II) Sulfide?
True or False: Ionic Solids have low melting and boiling points.
What is false?
Draw the lewis dot structure for Calcium chloride.
TRUE OR FALSE: An ionic bond is an electrostatic force that holds together oppositely charged atoms in a compound
What is true?
What is the correct formula for the compound formed between beryllium and nitrogen?
What is Be3N2?
Which is the cation in the compound CoCO3?
What is Cobalt?
When a potassium atom reacts with bromine, the potassium atom will...
what is lose an electron?
Draw the lewis structure for aluminum chloride
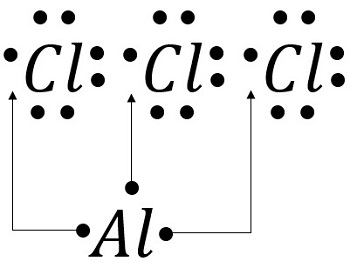
What is the correct name for the compound
What is ammonium sulfate?
Which is the correct formula for the compound Maganese (III) Fluoride?
Name this compound: Ca(OH)2
Calcium hydroxide
An ionic bond is formed when....
electrons in the valence shell of one atom are transferred to the valence shell of another electron
What is the octet rule?
have 8 electrons in the outer ring
What is +3?
What is the relationship between metals and nonmetals in ionic bonding?
What is metals donate electrons to nonmetals?
What is (NH4)2CO3?
What is the correct name for the compound with the formula CrPO4?
What is Chromium (III) phosphate?
What is a polyatomic ion?
An ion that has multiple charges
TRUE OR FALSE: Metallic elements tend to form cations rather than anions.
TRUE
What is the oxidation number for the element represented in the picture?
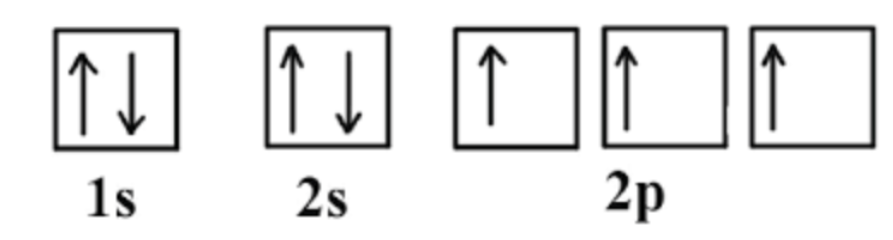
what is -3?
Which element would most likely form an ionic bond with chlorine?
-S
-K
-O
-N
What is potassium?
Correct name of the compound with the formula PbO2
What is lead (IV) oxide?
What does group 18 not bond with other elements?
Their outer shell is filled.