What color of light has the longest wavelength?
What is red?
What color of light has the longest wavelength?
What is red?
Covalent bond is sharing electrons between _____.
What is nonmental and nonmetal?
What is oxidation?
What is lose electrons
Define Electronegativity
What is the ability of an atom's ability to attract electrons?
As wavelength increases, energy ______
What is decreases?
Electrons need this to jump from one energy level to a higher energy level
What is photon or quanta of energy?
How many valence electrons does Phosphorus have?
What is 5?
What is reduction
what is gain electron?
Atomic radius increases _______ groups and decreases _______ periods.
What is down and across?
Which of the EM waves has the longest wavelength?
what is Radio waves
What is the element of this electron configuration?
1s2 2s2 2p6 3s2 3p3
What is Phosphorus ?
AlF3 has which type of bonding?
What is ionic bonding?
Which is more likely to lose it's electrons, Ca or Be?
What is Ca?
Which has larger atomic radius, Ba or Na?
What is Ba?
Compared to visible light, ultraviolet radiation is more harmful to the human being because ultraviolet radiation has a higher:
what is frequency?
What rule is being violated in this orbital diagram?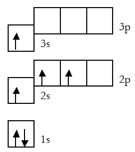
What is the Aufbau principle?
Use Lewis dot structures to show the covalent bonding for CO
(2)C(6)O(2)
Find the electronegativity difference between Rb and O.
What is 2.7 (3.5-0.8)?
Which has larger electronegativity, Ca or Cs?
What is Ca (less energy levels)?
Calculate the frequency of a light with a wavelength of 2.50x10-7 meters
f=c/wavelength
1.2x1015 Hertz
This the name of the state where the electron exists before being moved to a higher energy level
What is ground state
What is the Lewis dot structure for Al3+
What is Al?
Which of the following elements has the smallest atomic radius: Cs, Ra, F, or Rn
What is Cs (cesium)?