A type of matter formed by chemically joining two or more elements
What is a compound?
Boiling point, solubility, color, taste and density are all examples of this.
What are physical properties?
This is how particles in a solid move.
Cutting a piece of paper in half.
What is physical change?
An object has a mass of 15 g and a volume of 4.5 cm3. Find its density.
What is 3.33 g/cm3?
Hydrogen, Gold, and Iron are all examples of this.
What are elements?
A sheet of metal is 2 cm wide, 10 cm tall and 1.5 cm long. It has a mass of 42 grams. This is its density.
What is 1.4 g/cm3?
The most common state of matter in the universe is this.
What is plasma?
Burning a candle.
What is chemical?
Bose-Einstein condensates are used to simulate conditions in these.
What is blackholes?
This is another name for a homogeneous mixture
What is solution?
This property of matter measures a substance’s ability to dissolve.
What is solubility?
The amount of energy needed to change a material from the solid state to the liquid state is called this.
What is heat of fusion?
Melting a candle.
What is physical?
List two examples of where plasma can be found.
What are neon lights and the sun (stars)?
This is the difference between compounds and mixtures.
What is compounds are chemically combined, mixtures are physically combined?
Aluminum has a density of 2.7 g/mL. When a piece of it is placed in a graduated cylinder with 11 mL of water, the water rises to 20 mL. Find the mass of the aluminum.
What is 24.3 grams?
Matter that has a fixed volume but no fixed shape is this.
What is a liquid?
Bike wheels rusting.
What is chemical?
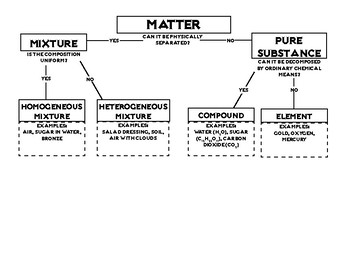
Draw the flowchart used for classifying matter.
Determine if the following mixtures are homogeneous or heterogeneous:
1.Saltwater
2.Vegetable soup
3.Milk
1.Homogeneous
2.Heterogeneous
3. Homoegeneous
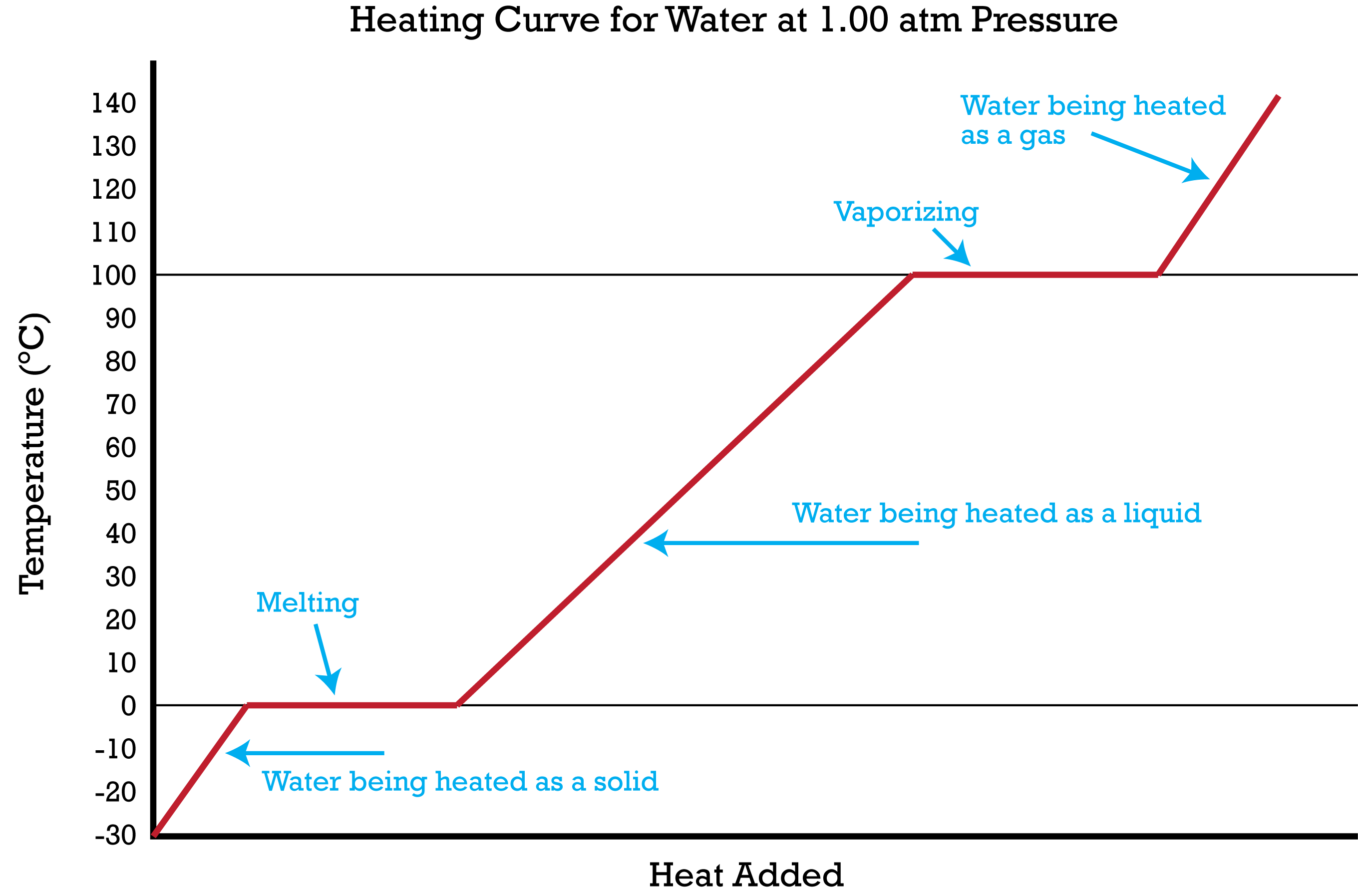
Sketch a heating curve and label the parts.
Think about the changing states of matter diagram. Write the names for the following transitions:
1.Gas -> Liquid
2.Liquid -> Solid
3.Gas -> Solid
1.Condensation
2.Freezing
3.Deposition
Steam coming off a shower.
What is physical?
List 3 of the signs that a chemical reaction has occurred.
Release of light
Temperature change
Odor change
Sudden color change
Gas is given off
Sudden appearance of a solid (called a precipitate)