element or compound
WATER

Compound
How many elements are there in the equation: He2CHCl
4
Increasing this factor makes molecules move faster, speeding up dissolution.
Temperature
Cutting paper into smaller pieces is an example of this type of change.
Physical
a part of a solution that dissolves in another substance.
Solute
This is the smallest unit of an element that still retains its properties.
What is an atom?
In the chemical formula H₂O, the "2" tells us this.
What is the number of hydrogen atoms.
Stirring or shaking a solution speeds up dissolving because it does this.
increases particle collisions
When is our Unit 6 test?
Tomorrow!!!!
The substance that does the dissolving in a solution.
Solvent
A pure substance made of only one kind of atom.
What is an element
2CH2OHP
C=2
H=6
O=2
P=2
Crushing a solute into smaller pieces affects this factor of dissolution.
surface area
Name evidence that a chemical change has occurred.
What are gas production,change of smell, color change,or a new substance forming.
The term for how much solute is dissolved in a given amount of solvent.
What is concentration
Is this an Element or a Compound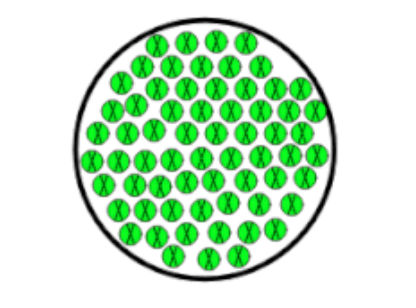
Element
4CHO(O)2
C=4
H=4
O=12
A sugar cube dissolves slower than granulated sugar because of this factor.
surface area
Melting an ice cube and dissolving sugar in water are examples of this change.
physical change
You make a cup of hot chocolate by mixing cocoa powder into hot milk. Your little sibling says, "Wow, the milk disappeared!" Explain to them why that’s not what happened, using the correct scientific terms.
What is the milk is the solvent, the cocoa powder is the solute, and together they form a solution—no magic involved, just science?
This is the main difference between elements and compounds when looking at chemical symbols and formulas.
Elements have one capital letter, while compounds have multiple elements represented by chemical formulas.
The formula Al₂(SO₄)₃ represents aluminum sulfate. How many atoms of each element are present in this compound?
2 aluminum (Al), 3 sulfur (S), and 12 oxygen (O)
Rank the three factors (temperature, surface area, agitation) from most to least effective in increasing the rate of dissolution.
1. temperature
2. agitation
3. surface area
Rust forming on iron is an example of this type of change.
Chemical
If you add more water to a solution, you are doing this to it.
Diluting/ dissolve