Identify the type of reaction:
Decomposition
![]()
58.44 g/mol
How many moles of O2 are needed to react with 0.512 moles of Al? (Hint: You don't need to find the LR!)
0.384 mol O2
Zn (s) + Sr(NO3)2 (aq) --> ?
No!

Soluble
Identify the type of reaction:
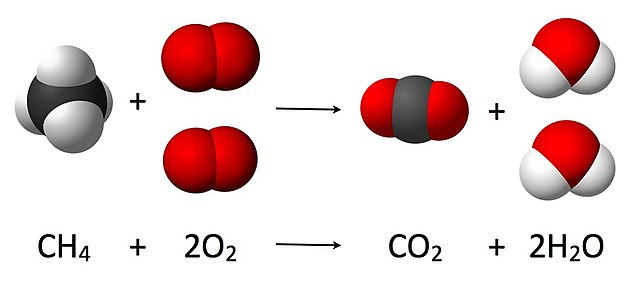
Combustion

159.808 g/mol
15.3g of Al2O3 were produced in this reaction. How many moles of O2 did the reaction start with? (Hint: You do not need to find the LR)
0.225mol O2
Cu (s) + PtCl2 (aq) --> ?
Yes!

Insoluble
Identify the type of reaction:

151.908 g/mol
How many grams of Al2O3 will be produced if 52.34 g of Al and 37.66g of O2 react? (Start by finding the LR)
80.0 g Al2O3
Br (g) + SF6 (aq) --> ?
No!

Soluble
Identify the type of reaction:
Double Replacement

158.16 g/mol
DAILY DOUBLE! 56.4g of Al2O3 were produced. How many grams of Al were reacted? (Hint: You do not need to find LR)
29.85g Al
Cd (s) + Pb(NO3)4 (aq) --> ?
Yes!
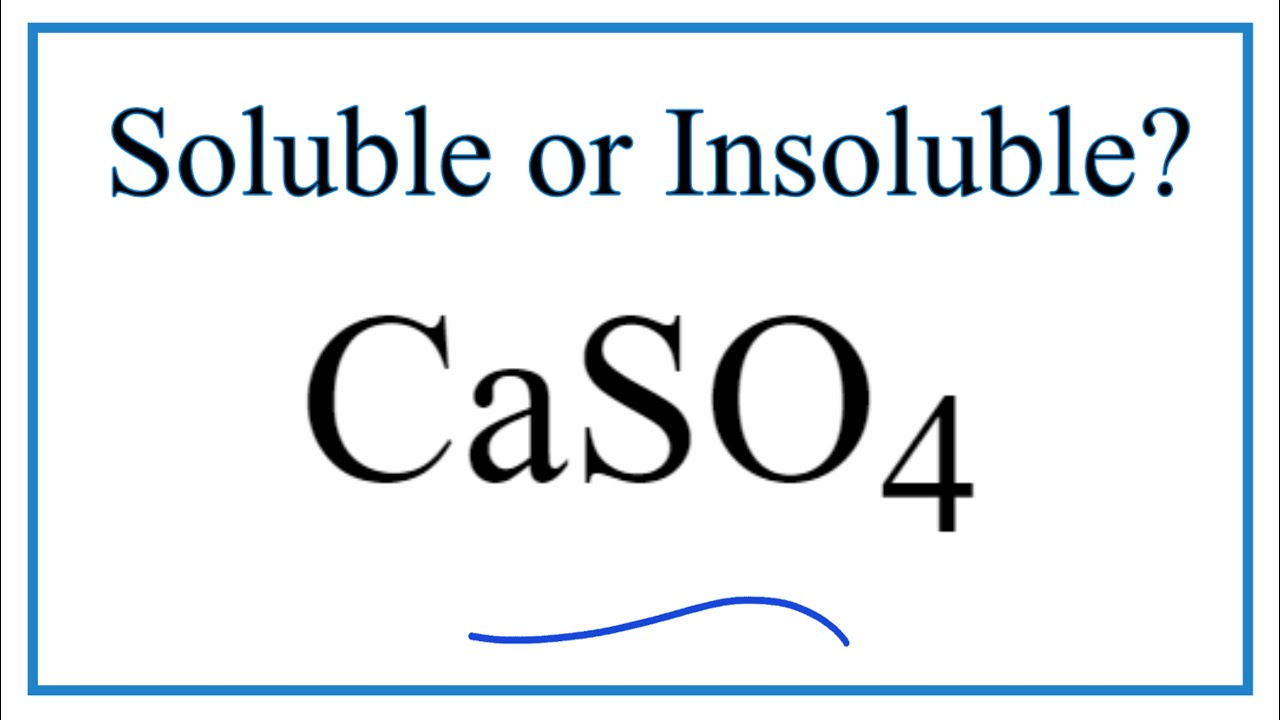
Insoluble
Identify the type of reaction:

Metallic Single Replacement

96.09 g/mol
How many moles of Al2O3 will be produced if 52.34g of Al and 37.66 of O2 react? (Start by finding the LR)
0.785 mol
Sb (s) + HgNO3 (aq) --> ?
Yes!

Insoluble