Drink
What is the enthalpy for the following goal equation?
PCl5(g) → PCl3(g) + Cl2(g)
Using the following information:
| P4(s) + 6Cl2(g) → 4PCl3(g) ΔH = -2439 kJ
| 4PCl5(g) → P4(s) + 10Cl2(g) ΔH = 3438 kJ |
What is 249.8 kJ
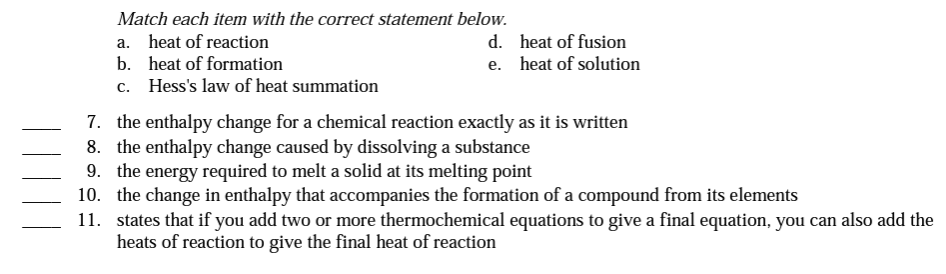
The value of the ΔH for any reaction that can be written in steps equals the _____of the value of ΔH for each of the individual steps
What is sum
What is the maximum amount of energy that could be produced from the reaction of 85.0 grams of Aluminum with 95.0 grams of oxygen, according to the following reaction?
4 Al + 3 O2 -> 2 Al2O3 H = -3352 kJ
What is -2638.14 or -2640 kJ
Aluminum is the limiting reactant
Is ΔH positive or negative in an exothermic reaction?
What is negative.
How much heat, in joules, would be required to raise the temperature of 450 g of Aluminum (0.900 J/g0C) from 19.50C to 31.20C?
4738.5


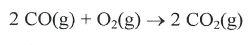

How many J would it take to raise the temperature of 200 grams of water from 5 oC to 85 oC?
Q = (200 g)(4.18)(80 oC) = 66880 J

B
C
E
F
A
D
E
Hunstville
Montgomery
Birmingham
Alabama
Aerosmith
Which country invented Gazpacho?
Spain

Samuel L Jackson

Elphaba
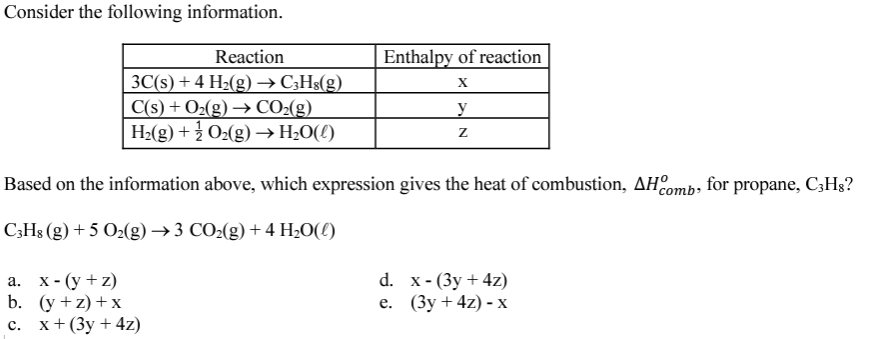
What is the change in enthalpy for the following goal equation?
CS2 + 2 H2O --> CO2 + 2 H2S
Given the following information:
H2S + 3/2 O2 --> H2O + SO2 ΔH = -563 kJ
CS2 + 3 O2 --> CO2 + 2 SO2 ΔH = -1075 kJ
What is 51 kJ
When an equation is reversed- the sign of ΔH must also be _________________.
What is reversed
How much heat will be transferred when 0.54g of sulfur reacts with 0.54g of oxygen to produce sulfur trioxide according to the following reaction:
2 S + 3 O2 -> 2 SO3 + 790kJ
What is -4.4 kJ
Oxygen is the limiting reactant
On a sunny spring day, while walking on the sidewalk near your house, you notice a puddle. Next to the puddle you see a fire hydrant. Which one is warmer to the touch? Do you know why? Assume each object received the same amount of sunlight.
Hydrant is warmer because it is made of metal. Metals conduct heat better than water. (Specific heat is metal is lower than water)
How much heat (in Joules) is needed to raise the temperature of 257g of ethanol (cethanol=2.4 J/g°C) by 49.1°C
30284.88 Joules
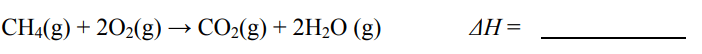

What would the final temperature be if 500 J are applied to 150 grams of ice at –90 oC?
500 J = (150 g)(2.06)(x – (-90 oC)) =-88.4 oC
A
E
D
B
C
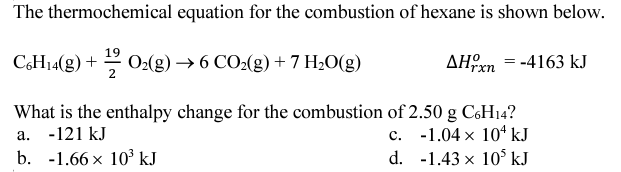
A
Little Rock
Fayetteville
Arkansas
Lady Gaga
Kimchi is a popular side dish from where?
Korea
Denzel Washington
Johnny Depp

Glinda
What is the change in enthalpy for this goal equation?
NO + O --> NO2
Given the following information:
2 O3 --> 3 O2 ∆H = -427 kJ
O2 --> 2 O ∆H = +495 kJ
NO + O3 --> NO2 + O2 ∆H = -199 kJ
What is -233 kJ
If all coefficients of an equation are multiplied or divided by the same factor, the value of ΔH must likewise be ______________________.
What is multiplied or divided by the same factor
How much heat will be absorbed when 38.2 g of bromine reacts with 12.4g of hydrogen according to the following equation? Which is the limiting reactant?
H2 + Br2 + 72.80 -> 2HBr
What is +17.4 kJ
Bromine
In an endothermic reaction, is heat gained or lost in the system? Draw a graph to illustrate the transfer of energy in an exothermic reaction.
What is gained, reactants--> products. Graph should show energy increasing in the system (solution gains/absorbs heat)
What mass of iron (ciron= 0.11 cal/g°C) would need 1450 cal of energy in order to raise its temperature by 19.7°C
670 g


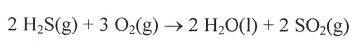


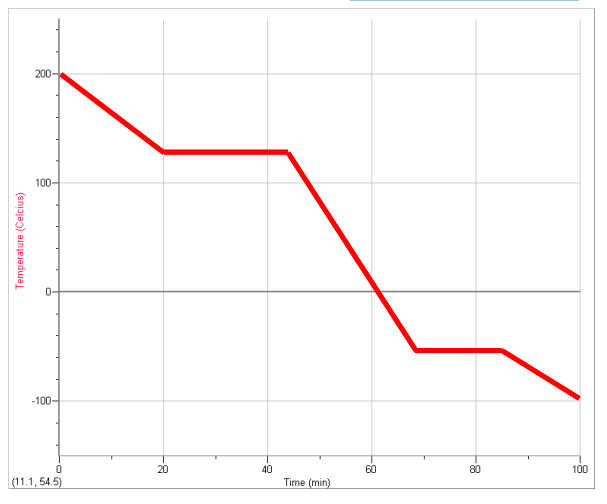
Octane, the major component in gasoline freezes at –57 oC and boils at 125 oC. If gaseous octane was cooled from 200 oC to –100 oC, draw what the graph would look like:

B
C
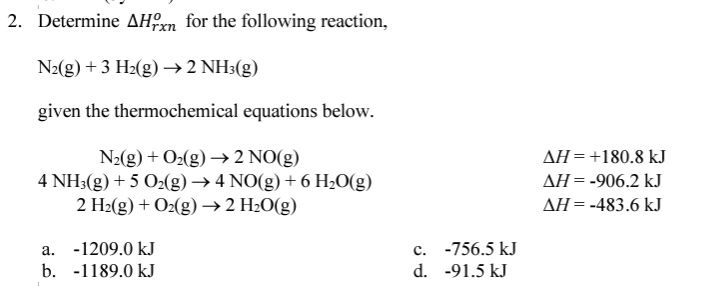
D
Bridgeport
Stamford
New Haven
Connecticut

Green Day
What is the name of a coffee drink prepared by diluting espresso with water?
Americano

Brad Pitt
George Clooney
Gary Oldman

Fiyero
What is the change in enthalpy for the following goal equation?
H2SO4(l) → SO3(g) + H2O(g)
Using the following information:
| H2S(g) + 2O2(g) → H2SO4(l) ΔH = -235.5 kJ
| H2S(g) + 2O2(g) → SO3(g) + H2O(l) ΔH = -207 kJ
| H2O(l) → H2O(g) ΔH = 44 kJ |
What is 72 kJ
When the given standard enthalpy formulas are reversed or "flipped," what must you do to the given change in enthalpy (delta H)?
change the sign
How much energy is released when 67.04g of phosphorous is reacted with 10.20g of chlorine? Which is the limiting reactant?
__ P + __ Cl2 --> __ PCl3 H = -574 kJ
What is -27.53 kJ
The Cl2 was the limiting reactant
A sample of ethanol absorbs 23.4kJ of energy. The temperature increases from 5.6 degrees Celsius to 19.8 degrees Celsius. What is the mass of the sample if the specific heat capacity of ethanol is 2.46 J/ (goC)?
What is 669.87 grams
What is the final temperature of a samples of nickel (cnickel= 0.54 J/g°C) if 328 J of energy is added to a 16.7g sample at an initial temperature of 24.4°C?
What is 60 degrees Celsius


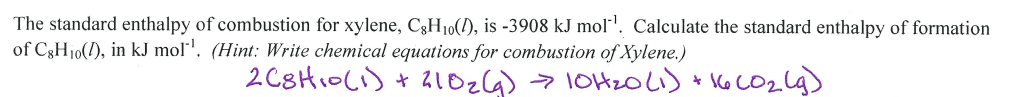
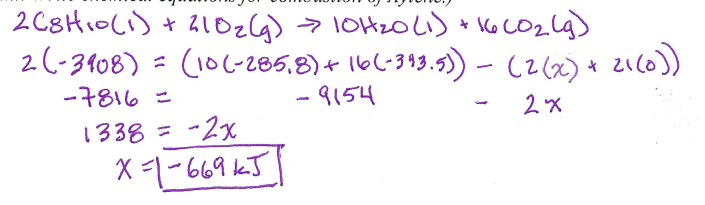
When 100 grams of hot water at 80 oC is combined with 100 grams of cool water at 20 oC. What is the final temperature of the combined water?
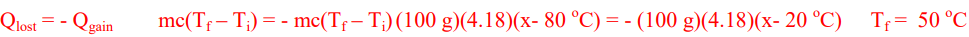

D
B
C
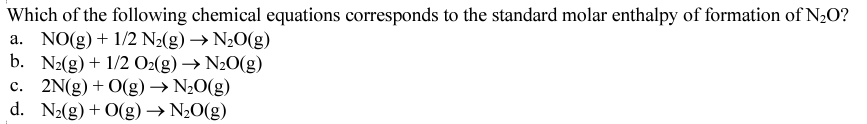
B
Des Moines
Cedar Rapids
Davenport
Iowa
Alicia Keys
What does ‘Carne Asada’ mean in Spanish?
Grilled Beef

Julia Roberts
Halle Berry
Angelina Jolie
Charlize Theron

Boq
What is the change in enthalpy for the following reaction?
1/2H2(g) + 1/2Cl2(g) → HCl(g)
Given the following standard enthalpies:
| COCl2(g) + H2O(l) → CH2Cl2(l) + O2(g) ΔH = 47.5 kJ
| 2HCl(g) + 1/2O2(g) → H2O(l) + Cl2(g) ΔH = 105 kJ
| CH2Cl2(l) + H2(g) + 3/2O2(g) → COCl2(g) + 2H 2O(l) ΔH = -402.5 kJ|
What is -230 kJ
The enthalpy change of a physical or chemical process depends only on the beginning conditions (reactants) and end conditions (products). The enthalpy change is __________ (dependent/independent) of the pathway of the process and the number of intermediate steps in the process
What is independent
How much energy would be produced from the reaction of 2.40 moles hydrogen with 3.95 moles chlorine? Which is the limiting reactant?
__ H2 + __ Cl2 ---> __ HCl H = -554 kJ
What is -1330 kJ
H2 is the limiting reactant
H2SO4(aq) + 2NaOH(aq) --> Na2SO4 (aq) + 2H2O (l) + 114 kJ (a)What is the enthalpy change for the given reaction? (b)How much energy would be given off if there were 31.2 grams of water produced?
What is (a) -114 kJ (b) -98.8 kJ of heat or 98,800 J
What is the specific heat of an unknown metal if 1.67 kJ of energy are required to raise the temperature of 79.2 g sample of the metal by 63.3°C
What is 0.333 J/g*C
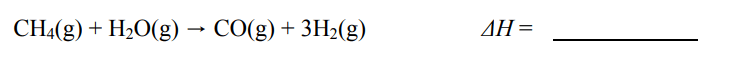
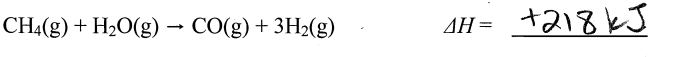
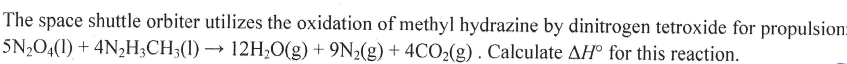
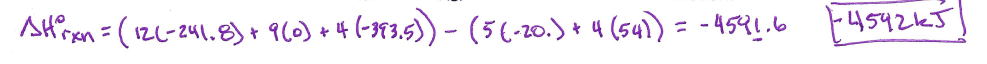
A 9.36 g piece of Pt is heated to 98.6 oC and then dropped onto a block of ice. When the temperature of the metal has dropped to 0 oC, it is found that 0.37 g of ice melted. What is the specific heat capacity of Pt?
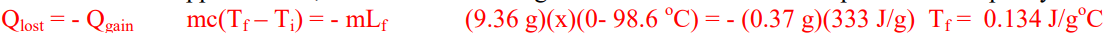

D
D
B
A
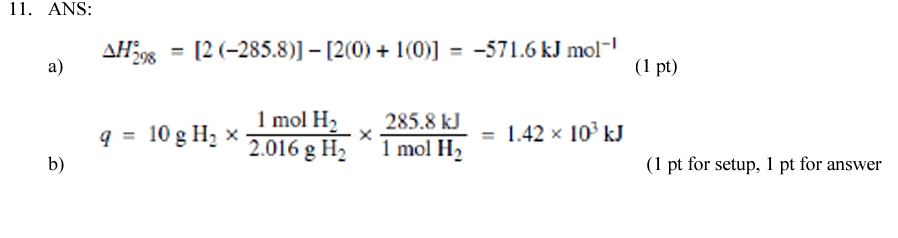
Portland
Lewis
Bangor
Maine

Linkin Park
What is Sauerkraut made out of?
Cabbage

John Krazinski
Kelly Ripa
Bradley Cooper
Nicole Kidman
Jimmy Kimmel

Madam Morrible
