A positively charged ion.
What is a cation?
Bond which electrons are free floating as a sea of shared electrons.
Metallic bond
Two electron domains
Zero lone pairs
What is linear?
What is a nonpolar bond?
Chemical name of SCl2
Sulfur dichloride
When two atoms form a chemical bond together, each atom will usually attain a stable electron arrangement in their valence shells that contains this number of electrons.
Eight
The strongest type of bond
Ionic bond
Draw the Lewis Diagram of SeH2
SeH2
A bond with an electronegativity difference greater than 1.7
What is an ionic bond?
Chemical name of CrO
Chromium oxide
The primary factor in forming chemical bonds. This periodic trend increases left to right and decreases bottom to top.
What is electronegativity?
Bond which does not conduct electricity.
Covalent bond
Four electron domains
Zero lone pairs
Tetrahedral
Polarity?
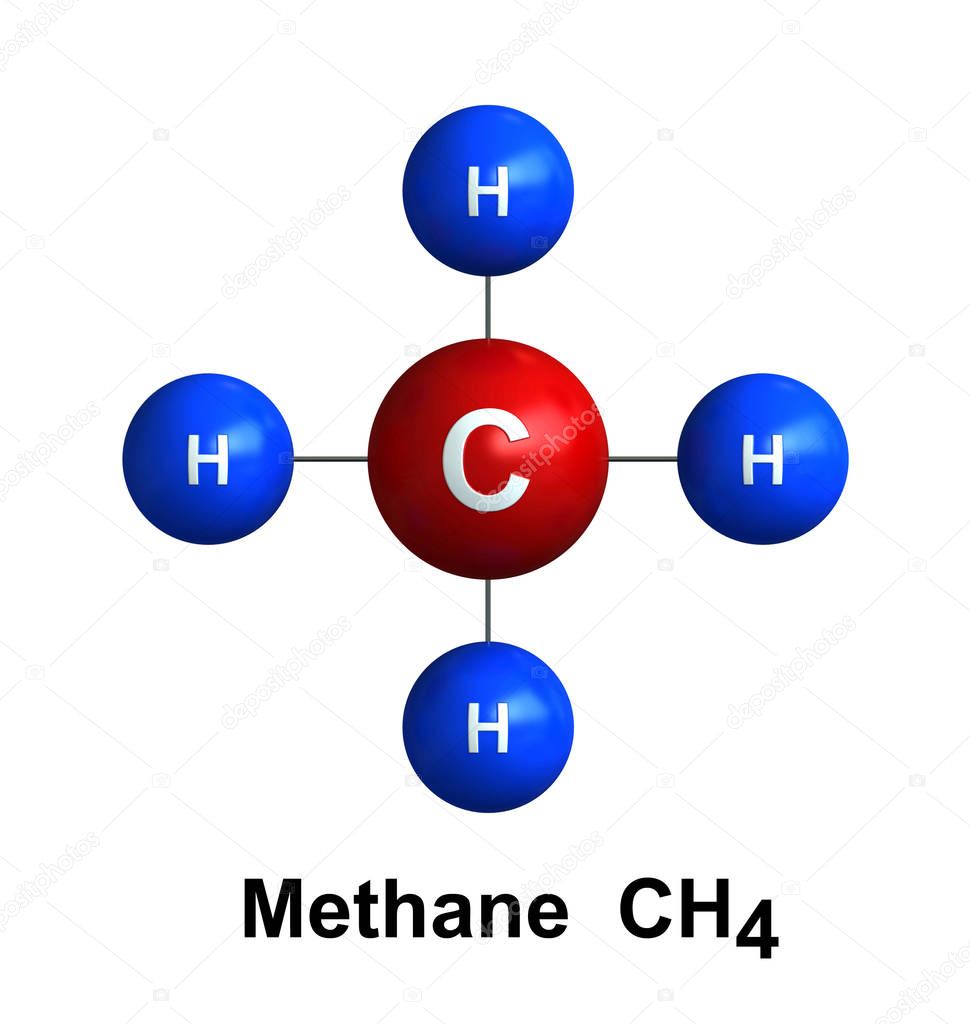
Nonpolar
Chemical formula for dinitrogen pentaoxide.
N2O5
Type of elements with low electronegativity (left of the staircase)
Metals
Can form single, double, or triple bonds.
Covalent bond
Four electron domains
Three shared pairs
one lone pairs
Trigonal pyramidal
Polarity of a Br--Cl bond
(Br=2.96) & (Cl=3.16)
0.2 Nonpolar bond
Chemical formula for hexacarbon decahydride
C6H10
Oxygen ion
O?
O2-
Ionic bond
Draw the Lewis Diagram for (CO3)2-
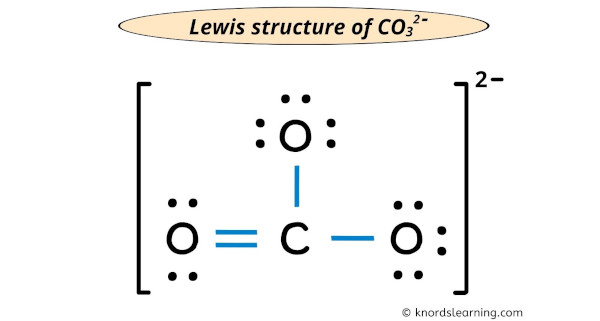 (CO3)2-
(CO3)2-
Polarity?
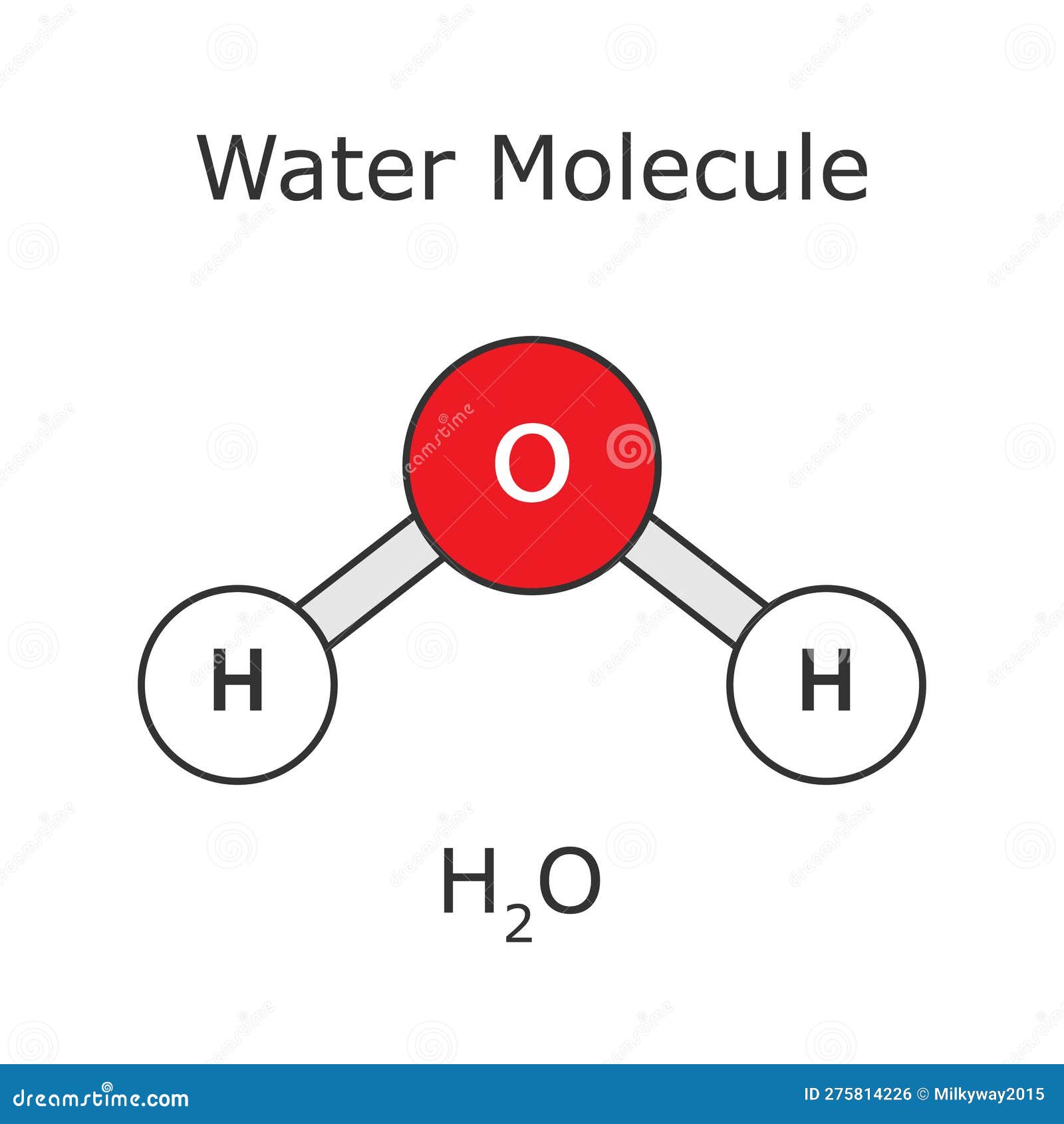
Polar
Chemical formula for an ionic compound of calcium and chlorine