 Based on the picture, where is the energy going?
Based on the picture, where is the energy going?
(Entering/Leaving) the system
Leaving the System
Based on the chart below that lists the specific heat, which of the following object will experience the least temperature change?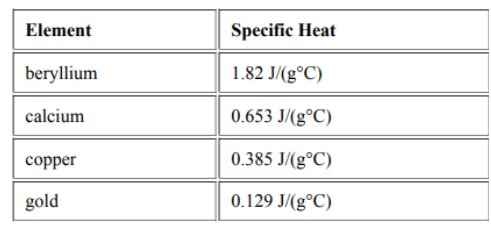
Berylium
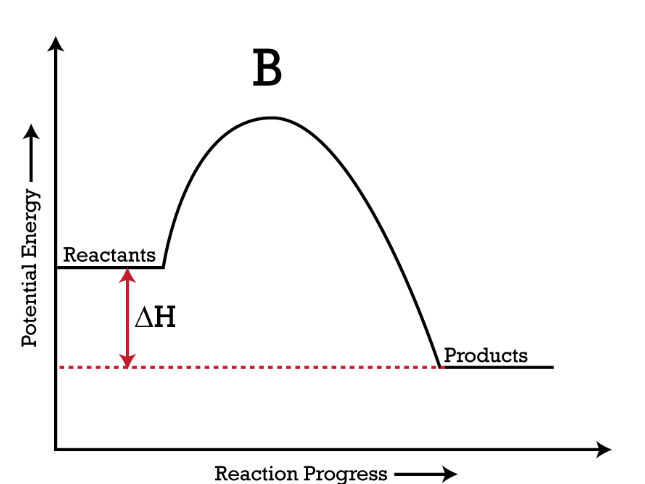
This is the heat content of a system at constant temperature and it is denoted as "ΔH "
How does heat spontaneously move from one object to another?
Heat moves from a hotter object to a cooler object.
Based on the picture, where is the energy going?
(Entering/Leaving) the system
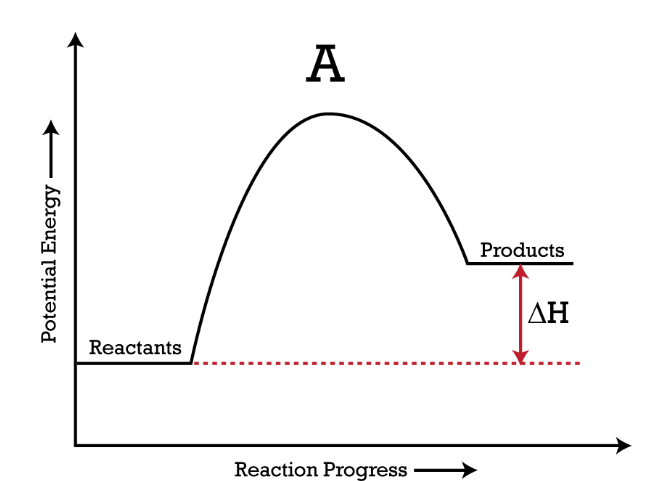
Entering the System
Rank the following objects from least to greatest regarding their temperature change when heated.
(Least Temperature Change --> Greatest Temperature Change)
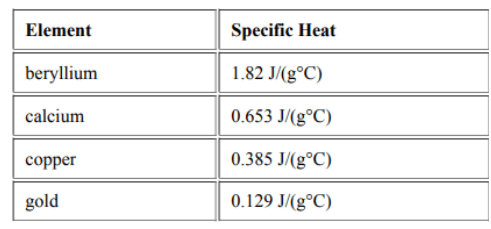
beryllium < calcium <copper < gold
This is a measure of the number of possible ways that the energy of a system can be distributed due to the randomness/disorder of particles in a system.
entropy
Determine the number of moles of 6.08 grams of SiO2.
(Hint: You need to use the periodic table for this to get the molar mass)
0.10 moles
Molar Mass: 60.08 g/mol
Based on the thermochemical equation, what kind of reaction will this be?

exothermic
Calculate the heat required to melt 205 g of Ice.
Given that Water's ΔHvap: 2257 J/g
Water's ΔHfus: 333.6 J/g
Values can be negative depending on the phase change
Useful Equations
q = mΔvaporization
q = mΔfusion
Answer is 68,400 J
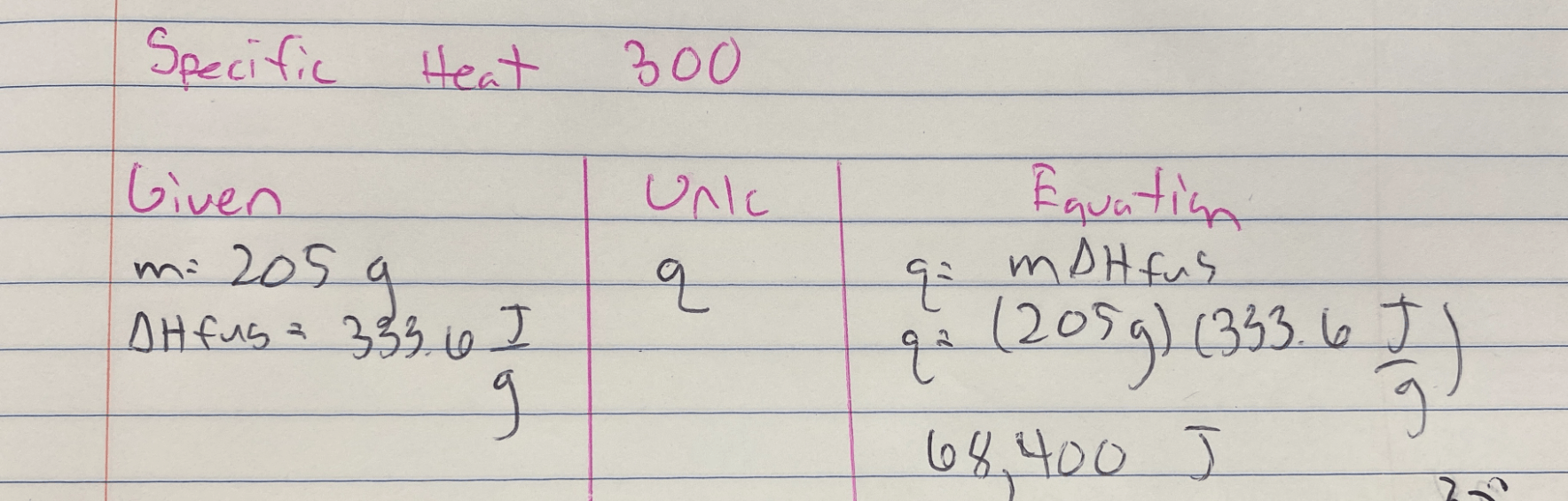
What are the two units of energy that we use for this unit?
Based on the thermochemical equation, what kind of reaction will this be?

Endothermic
How much energy being absorbed in Joules is required to heat 50.0 grams of iron with a temperature change of 55°C? The specific heat of iron is 0.444 J/g°C.
Useful Equations
q = mcΔT
Answer is 1220 J
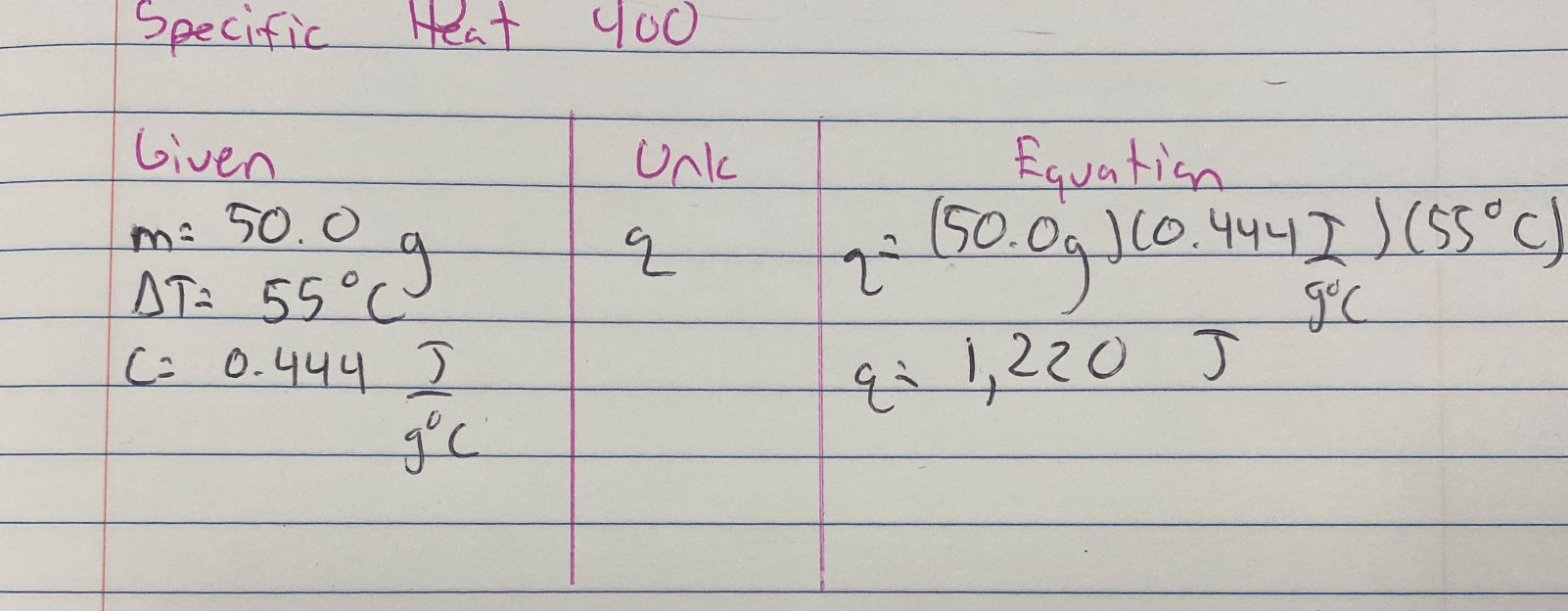
This is the heat content of a system at constant temperature and it is denoted as "ΔH "
Enthalpy
Please give one real life example of an exothermic and endothermic reaction
Answers might vary based on Teacher's discretion
How much energy is released when 25.0 grams of aluminum metal is cooled from 125°C to 35°C? The specific heat of aluminum is 0.900 J/g°C.
Useful Equations
q = mc(Tfinal - Tinitial)
Answer is -2030 J
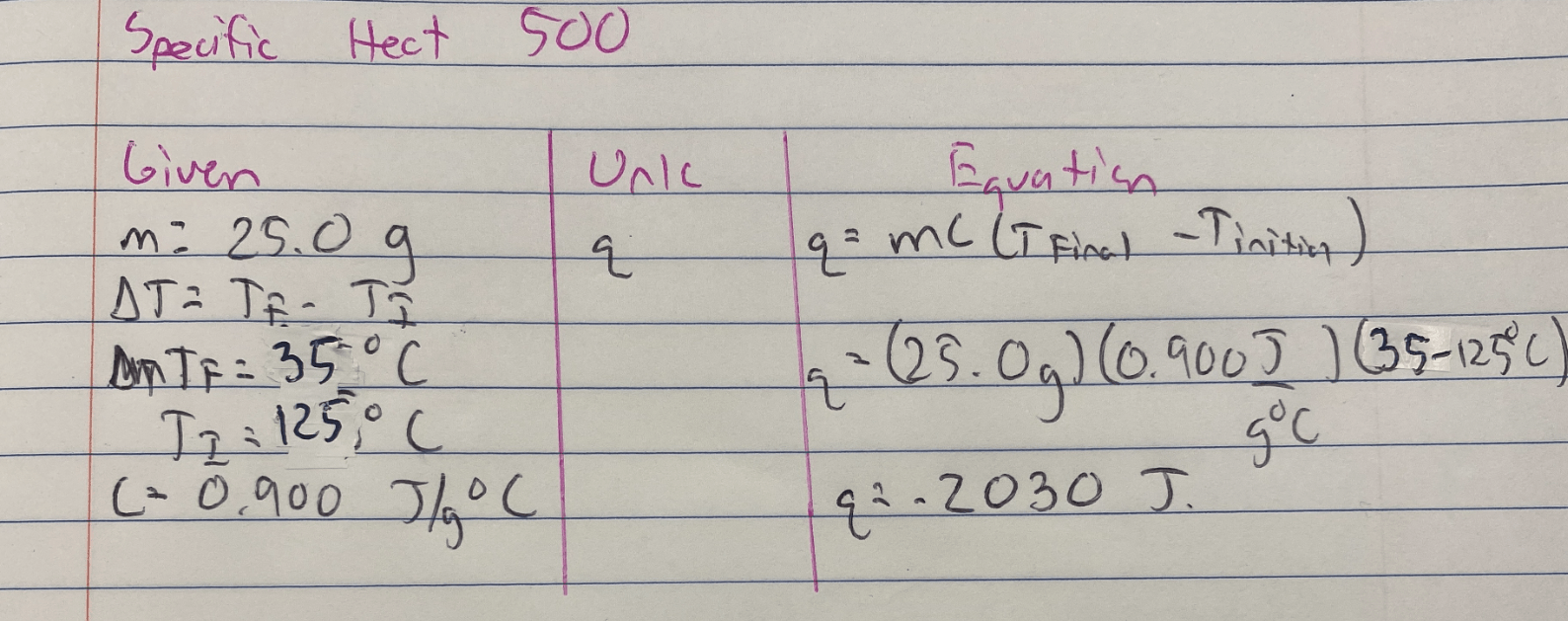
When we talk about the perspective about the heat exchange that is happening in the universe, what two things that it consists of?
System and Surrounding
Calculate the Standard Heat of Formation (ΔH) for this reaction. Is this exothermic or endothermic?
SO3 (g) + H2O (g) --> H2SO4 (aq)
Given that the ΔH of:
H2SO4: -909.27 kJ
H2O: -241.8 kJ
SO3: -395.7 kJ
Answer: -271.77 kJ (Exothermic)
Work:
H2SO4 ---> H2O + SO3
(-909.27) - (-241.8 + -395.7) = -271.77 kJ