What is the structure of the atom
protons, electrons, and neutrons. The nucleus of the atom contains the protons (positively charged) and the neutrons (no charge). The outermost regions of the atom are called electron shells and contain the electrons (negatively charged).
Types of mixtures
Homogeneous mixture is a system in which all components in the mixture form one phase (solid, liquid, gas)
Heterogeneous mixture is a system in which components can be identified. At least two or more different phases can be detected with the naked eye.
How many groups are in the periodic table?
8
the chemical symbol for Helium is
He
What is a molecule
Molecules are the result of the interaction between atoms, to be more specific, electrons of the last shell
What are the letters for Atomic Number and Atomic mass
A is the mass number and Z is the atomic number
distribute these types of mixtures
air
salt water
dirt
tea
coffee
solutions
pizza
- alloys of metals
- pulpy orange juice
- sandy water
Heterogeneous mixtures
pulpy orange juice
dirt
sandy water
pizza
Homogeneous mixture
air
salt water
tea
coffee
solutions
alloys of metals
where is the atomic mass?
12,011
definition for a chemical element
A chemical element is a substance consisting of only one type of atom
What is the difference between compound and Molecular elements?
An element is a substance that cannot be broken down further by chemical means. A compound is a molecule composed of different types of atoms bonded via chemical bonds.
What do Atomic mass mean? Describe it
Atomic number
The number of protons in the nucleus of an atom. Which is characteristic of a chemical element and determines its place in the periodic table.The number of protons define the identity of an element.
difference between Compound and Mixture
Compound - combination of two or more different elements via chemical bond. As a result of these combinations chemical identity of elements changes. For example: Sodium is a soft metal that we can cut with a knife. Chlorine is a poisonous gas, when they combine they create a new substance called table salt (sodium chloride) with different properties.
Mixture - combination of two or more substances with no chemical bond between them. As a result of these combinations chemical identity does not change. Example: sandy water consists of two substances, sand and water, if we sieve it, we can take pure water and sand.
What position are the lines and periods in?
Rows - Periods
Columns – Groups
What is the chemical symbol for Mercury
Hg
What is a chemical bond? (You should give an example)
Chemical bond - Molecules are formed thanks to the interaction between atoms.
EX:The bond between hydrogen and oxygen atoms to form water
Calculate, how many protons, electrons and neutron are here?
Protons: 20
Electrons:20
Neutrons: 20
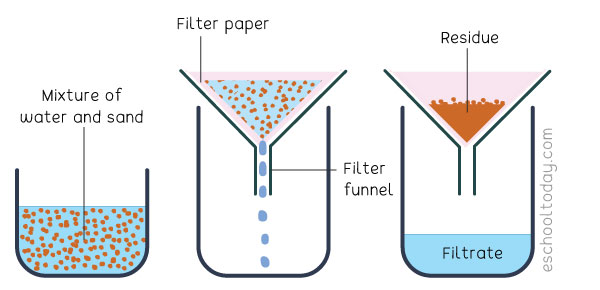
How does the filtration process work?
solid particles in a liquid or gaseous liquid are removed using filter paper, which allows the liquid to pass through but retains the solid particles.
What period is this atom in?
3,because

How is chromium read in chemistry?
Cr
what is the difference between negative and positive atomic ion?
If the atom has more electrons than protons, it is a negative ion, or ANION. If it has more protons than electrons,it is a positive ion.
Describe this atom
Atomic number: number of protons in an atom.In a neutral atom, the number of protons and number of electrons is the same. This atoms has 8 electrons, thus the atomic number is 8.
This element is placed in group number 6 in the periodic table
This element is placed in period 2 in the periodic table
The Robinson has sea salty water mixed with sand. How can you get salt? Describe it
1)Separate sand from sea water
by passing it through a porous medium (filter paper) that retains the solid but allows the liquid to pass
2)Separate salt from sea water
boil or evaporate the water and the salt will remain as a solid. Because salt has a much higher boiling point than water.
Boron, silicon, germanium, arsenic, antimony, and tellurium is
metalloids
an imprecise term used to describe a chemical element that forms a simple substance having properties intermediate between those of a typical metal and a typical nonmetal.

list 5 chemical elements and their symbols
For ex:

How are water and peroxide different?
Water is made of 2 hydrogen atoms and 1 oxygen atom. Hydrogen peroxide is made of 2 hydrogen atoms and 2 oxygen atoms