0.01 expressed in scientific notation
1 x 10-2
1.0003 has only this type of zero.
Sandwiched (Captive) Zeros
This is closeness of a measurement to an accepted value
accuracy
Convert 25.4 kg to grams.
25,400 grams
When measuring liquids in a graduated cylinder, it is important to measure from the bottom of the _________.
meniscus
1,200 written in scientific notation is
1.2 x 103
1,435,000 has this many significant figures
4 (trailing zeros before the decimal point are not significant)
reproducibility of measurements and agreement among numerical values best describes
precision
Convert 4.50 hours to seconds.
16,200 seconds
The length of this line in cm is 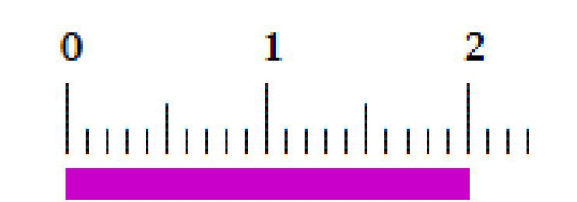
2.00 cm
5,678,900 written in scientific notation
5.6789 x 106
14.0 + 3.68 =
17.7 (lowest number of decimal places for +/-)
A group of students tried to guess Mr. Ly's weight. Their guesses were 165, 167, and 168 lbs. Mr. Ly's weight is actually 155 lbs. These guesses are not _________.
Accurate
Convert 16 lbs to kg
1 lb = 454 g
1 kg = 1,000 g
16lbs~~7.3kg
This graduated cylinder contains _____ mL of water.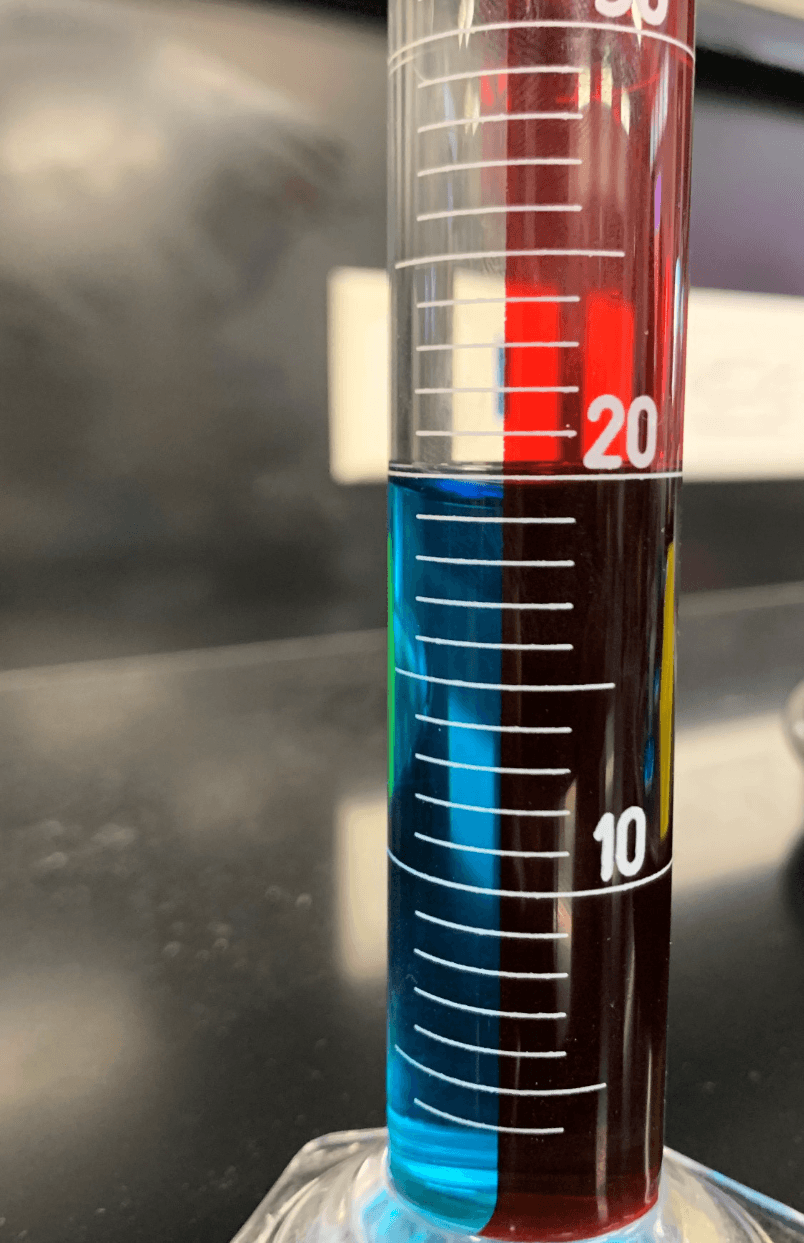
19.1, 19.2, or 19.3 mL
7.63 x 104 written in expanded form is
76,300
15 divide 3.0
5.0
A student experimentally determined the density of graphite to be 2.3 g/mL. The actual density of graphite is 2.23 g/mL. Calculate % error.
3.1%
Convert 1.25 quarts to L.
1 quart = 946 mL
1 L = 1,000 mL
1.25quarts~~1.18L
On a graph of mass v. volume, the slope of the best fit line represents _______.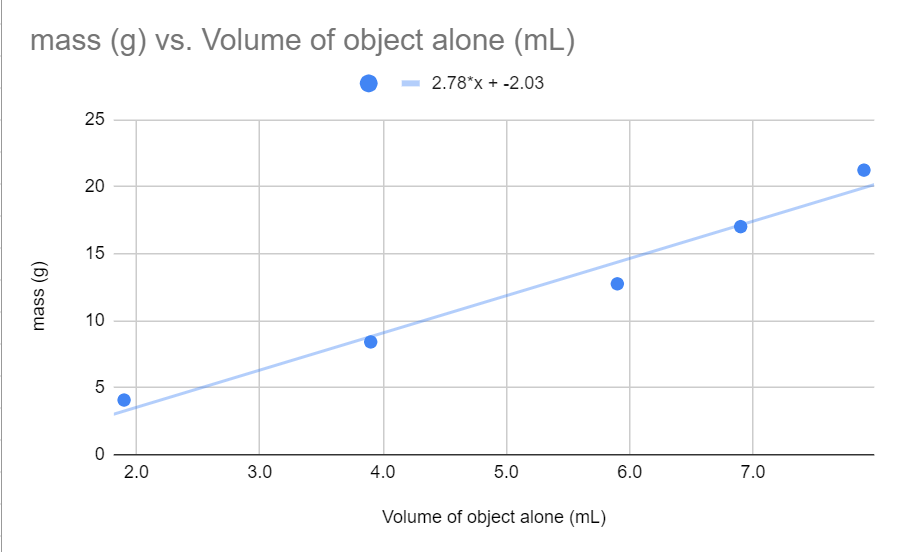
8.5201 x 10-7 written in expanded form is
0.00000085201
142 x 2.01
285
A football player that scores 10/10 field goals can be described as ______.
both accurate and precise.
Convert 207 kg/dL to g/mL.
1 kg = 1,000 g; 1 L = 10 dL;
1,000 mL = 1 L
2,070 g/mL
This laboratory method is used to determine the density of an object by fully submerging it in water and measuring the volume change.
Water displacement