This is the highest point of a wave.
What is the peak?
This type of wave requires a medium.
What is a mechanical wave?
Of the colors in the visible spectrum, ROYGBIV, this color has the lowest frequency.
What is red?
Both fire and electricity are adding this to the atoms during the flame test and the emission tube experiments.
What is energy?
The line from the image below with the least amount of energy.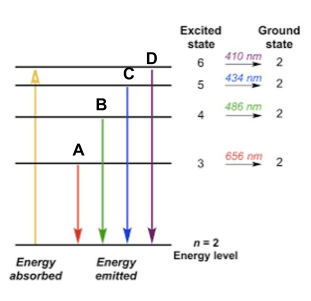
What is A?
Valence electrons are located here.
What is the outermost energy level?
This is the distance between two peaks of a wave.
What is the wavelength?
This type of wave does not require a medium.
What is an electromechanical wave?
Of the colors in the visible spectrum, ROYGBIV, this color has the highest energy.
What is violet or purple?
This is a unique observation for each element when energy is added to excite the atoms.
What is the emission spectrum?
The line from the picture below with the greatest amount of energy.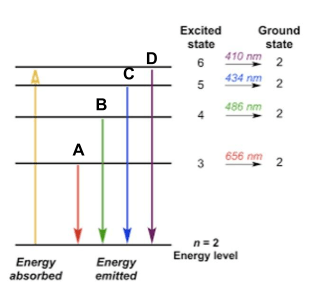
What is D?
The first energy level can hold this many electrons.
What is 2?
This measurement is represented by the symbol
nu
What is frequency?
A cell phone wave, which is a radio wave, has this type of wavelength: long or short.
What is a long wavelength?
The nm unit used to measure the wavelength of light stands for this.
What is nanometers?
The different colors in an element's emission spectrum represent these.
The yellow arrow represents this in the picture below.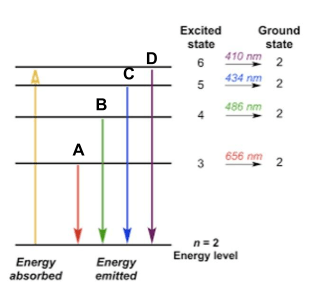
What is the electron being excited to a higher energy level?
A full energy level, normally containing 8 electrons, is called this.
What is a full octet?
This measurement is represented by the symbol
What is wavelength?
Violet (purple) light has this type of wavelength: long or short.
What is a short wavelength?
When you looked at sunlight with the spectroscope you saw this kind of spectrum.
What is a continuous spectrum or a rainbow?
Colors with a high frequency indicate energy levels that are either this: far from the nucleus or close to the nucleus.
What is far from the nucleus?
The color of light emitted when an electron drops from the 6th energy level to the 2nd energy level.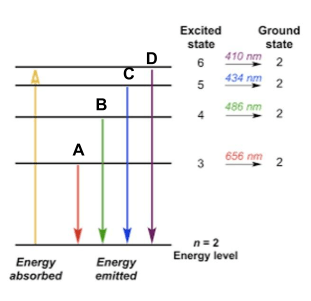
What is purple?
Nitrogen has this many valence electrons.
What is 5?
This is the number of waves that pass by in one second.
What is frequency?
When the wavelength of an electromagnetic wave increases, this happens to its frequency.
What is decreases?
When you looked at the gas emission tubes through the spectroscopes you saw this type of spectrum.
What is an emission spectrum or lines of colors?
This is what causes the colors to be emitted when energy is added to an atom.
What is energy is added to the electron causing it to move to a higher energy level. When the electron returns to the ground state it emits that energy as light of a certain frequency.
The color of light emitted when an electron drops from the 4th energy level to the 2nd energy level.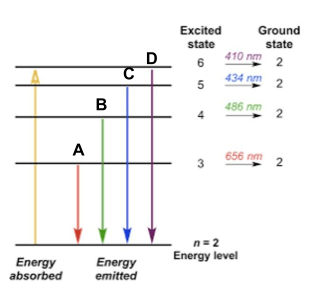
What is green?
Noble gases have this many valence electrons?
What is 8?