Before leaving the lab area, one should do this to their hands.
What is washing/sanitizing your hands?
The CMS-116 Form can be used to update this. This should also be visibly posted in the Lab area at all times. 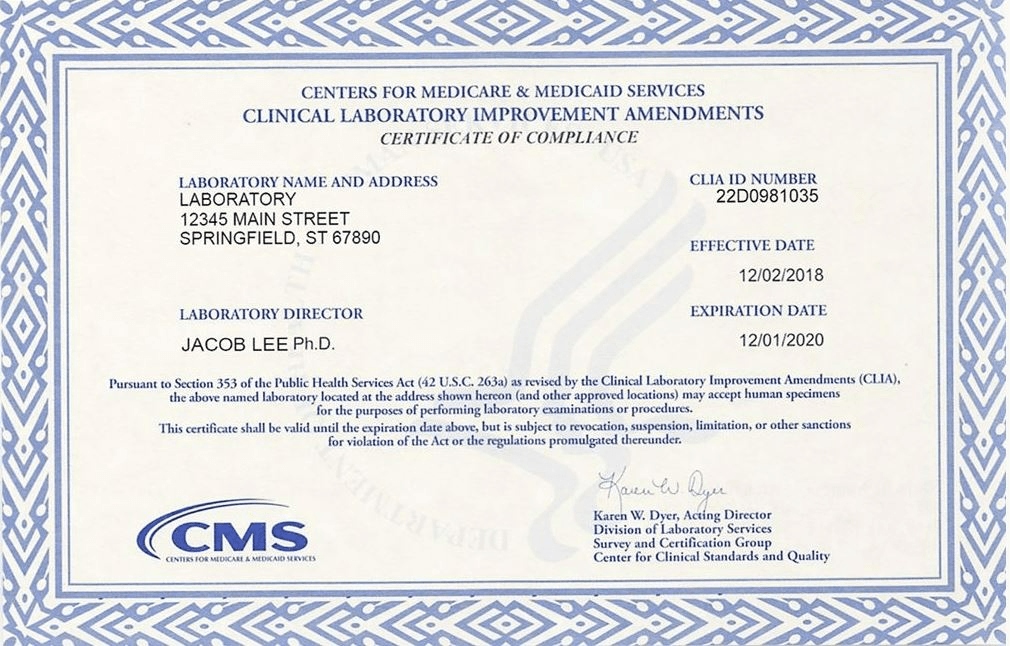
What is a CLIA Certificate?
All Quality Control should be documented on this type of log, shown below.
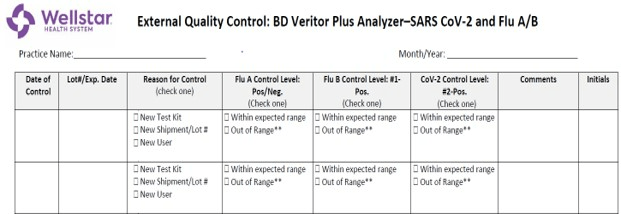
What is a QC Log?
Providing _____ and ________ on all documentation is part of Good Documentation Practices.
What are Date and Initials?
Always use these while handling any patient samples and while disinfecting lab surfaces. This is known as Universal Biohazard Precautions.
What are gloves?
This should be done IMMEDIATELY after patient specimen collection.
What is Specimen Labeling?
Wearing gloves and a labcoat or PPE is best practice in the lab. PPE is short for this phrase.
What is Personal Protective Equipment?
CLIA inspections and certifications are controlled by this goverment entity.
What is CMS?
This type of control should be performed before using any testing kit or instrument.
What is QC (Quality Control)?
The BD Veritor uses these instead of test strips to test for different Respiratory diseases. You should not visually read these.
What are cassettes?
Using _______ is a good way of keeping track of different patient samples during testing. HINT: These print out after a patient is admitted.
What are labels? (Chart Labels)
Along with 2 UNIQUE patient identifiers, this should also be written on specimen collections.
What is the DATE OF DRAW/Collection?
These type of wash stations should be within 10 seconds of the lab area. These are for splash exposures to the eyes and face.
What are Eye Wash Stations?
Documenting Corrective Actions, like if an instrument fails or missing signature on a Log sheet, is a part of this type of Quality Plan.
What is the Quality Assurance Plan?
Quality Control for a CBC Analyzer should be performed every _____ hours. The amount of times is dependent on the operating hours of the facility.
What is every eight hours?
These test strips should be QC'd every day of patient testing.
What are Urinalysis (UA) test strips?
R.A.C.E. is another acronym for fire safety and stands for this.
What is Rescue, Activate, Contain, Extinguish.
Your _______ should also be written on specimen collections, along with the 2 Patient IDs.
What is your initials?
Stop. Think. Ask. Review. or this acronym for short, is a great mnemonic device and best practice to maintain Patient Safety at work.
What is S.T.A.R?
The Lab Manual is filled with these that detail how to do everything in the lab. All lab personnel and the lab director should provide yearly signatures acknowledging these.
What are Lab Policies?
Quality Control should be performed if you drop this device on the floor. This is a waived glucose testing device.
What is an EvenCare?
These inserts are useful when perfoming any waived test. The inserts are always included within the testing package and should be saved and kept in a binder on-site.
What are Package Inserts?
DAILY DOUBLE!!!
To be compliant with ________ rules, patient information should be removed or redacted (black marker strikeout) before disposing of patient samples.
What is HIPPA?
TRUE or FALSE.
This is an example of proper specimen labeling.
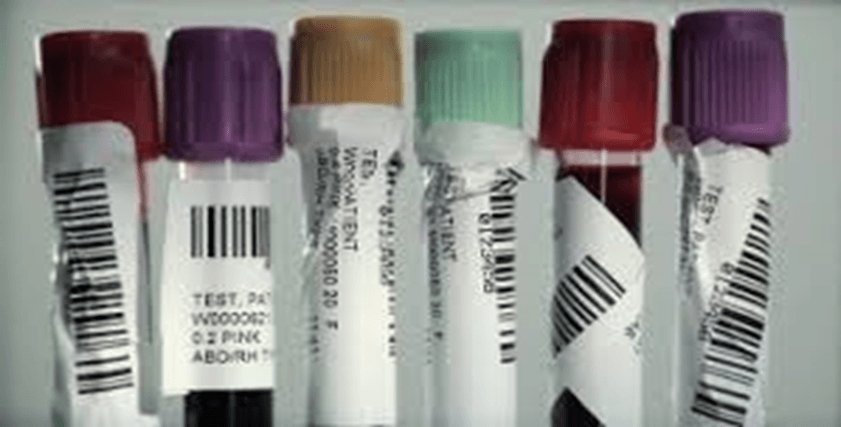
FALSE
Lab testing personnel should use at least 2 of these to confirm patient samples prior to testing.
What are two personal identifiers?
This CMS form can be used to track all testing personnel who perform testing in the laboratory. This is especially true for non-waived (moderate to high complexity) Testing.
What is the CMS-209 Form or Competency Tracker?
If QC fails on the first attempt, the testing personnel should do this before using the instrument or kit for patient resulting.
What is Repeat the QC?
Testing personnel uses the ___________ device to read the results for the Clinitest hCG Pregnancy Test. Visual reads of hCG cassettes is not advised.
What is the Clinitek?
DAILY DOUBLE!!!
TRUE or FALSE
PPE or any material that is not visibly soiled with bodily fluids can go in the regular trash.
What is True?
TRUE or FALSE.
Improper Specimen labeling can cause misidentification of patient samples, lost specimens and hinder sample testing in the laboratory.
TRUE!