Anything that has mass and takes up space.
Matter
Building blocks of matter.
Atoms
State of matter that has a specific shape and specific volume.
Solid
Materials that allow energy to pass through them easily.
Conductors
If water has a density of 1 g/mL, what will happen to an object of density 2 g/mL that is placed in water?
It will sink!
State of matter that will completely fill the space that it is given.
Gas
Each different type of atom.
Element
When a substance changes from a solid, to a liquid.
Melting
The ability of a solid to dissolve in a liquid.
Solubility
Which item in the column is the least dense?
Ping Pong Ball
State of matter that has particles that can move past each other. Takes the shape of its container.
Liquid
Two or more atoms chemically joined together.
Molecule (or Compound)
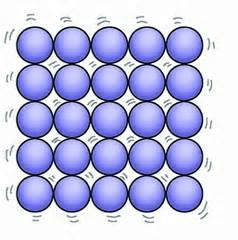
Solid
Name a metal that is magnetic.
iron
What must be true of the rubber duck?

It is less dense than water.
The amount of matter packed into a specific volume.
Density
How many different elements are in C6H12O6 ?
3
What it takes to change from one state of matter to another state of matter.
Energy (or Heat)
Which mineral(s) can topaz scratch?
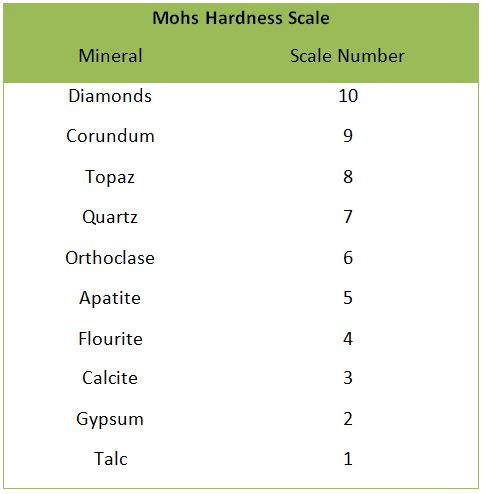
Quartz
Calculate the density of an object with a mass of 10g and a volume of 5mL.
Density = Mass/Volume
2 g/mL
State of matter that is similar to a gas, but has electrically charged particles.
Plasma
How many total atoms are in NaCl ?
2
When a solid changes directly to a gas!
Sublimation
Name 3 materials that would be considered insulators.
rubber, plastic, wood, glass...
Which item listed below would sink in water?
Item 1: density of 0.8 g/mL
Item 2: density of 1.06 g/mL
Item 3: density of 0.96 g/mL
Item 2