Gas
A state of matter with NO definite shape or volume
An ionic bond is formed between_?(a) two metal atoms,(b) two non-metal atoms ,(c) one metal atom and one non-metal atom,(d) one metal atom and one metalloid atom
(c) one metal atom and one non-metal atom
Name two metals that don't get corroded?
Gold(Au),and platinum(pt)
What is the electron configuration of sodium?
1s22s22p63s1 or [Ne]3s1
Mass Number
The total number of protons and neutrons in the nucleus of an atom.
soild
A state of matter that HAS a definite shape and a definite volume
The covalent compounds are soluble in? (a) all acids,(b) all bases,(c) all solvents,(d) non-polar solvents
(d) non-polar solvents
Identify the most reactive and least reactive metals:Al,K,Ca,Au
Most reactive metal:K(potassium),Least reactive metal:Au(gold)
What is the electron configuration of As?
1s22s22p63s23p64s23d104p3 or [Ar]4s23d104p3
Neutral Solution
an aqueous solution in which the concentrations of hydrogen and hydroxide ions are equal; it has a pH of 7.0
Solubility
A measure of how much solute can dissolve in a given solvent at a given temperature and pressure
Which of the following elements in group 17 is the most reactive?A.oxygen,b.sodium,C.fluorine,D.magnesium
C.fluorine
Respiration is an:A.exothermic reaction,B.endothermic reaction,C.Both a and B,D.none
A.exothermic reaction
What is the electron configuration of H?
1s1
what are the three classifications of matter?
Elements,compounds,and mixtures
Law of Conservation of Mass
law that states that the total mass of the reactants before a chemical reaction is the same as the total mass of the products after the chemical reaction.
Which of the following best describes the correct order of oxygen, fluorine, and nitrogen’s atomic radii?A.(O < F < N),B.(N < F < O), C.(F < O < N), D.(O < N < F)
C.(F < O < N)
BI
What is the electron configuration of neon?
1s22s22p6 or [He]2s22p6
what is the ratio of mass to volume of a substance?
density
Beta Particle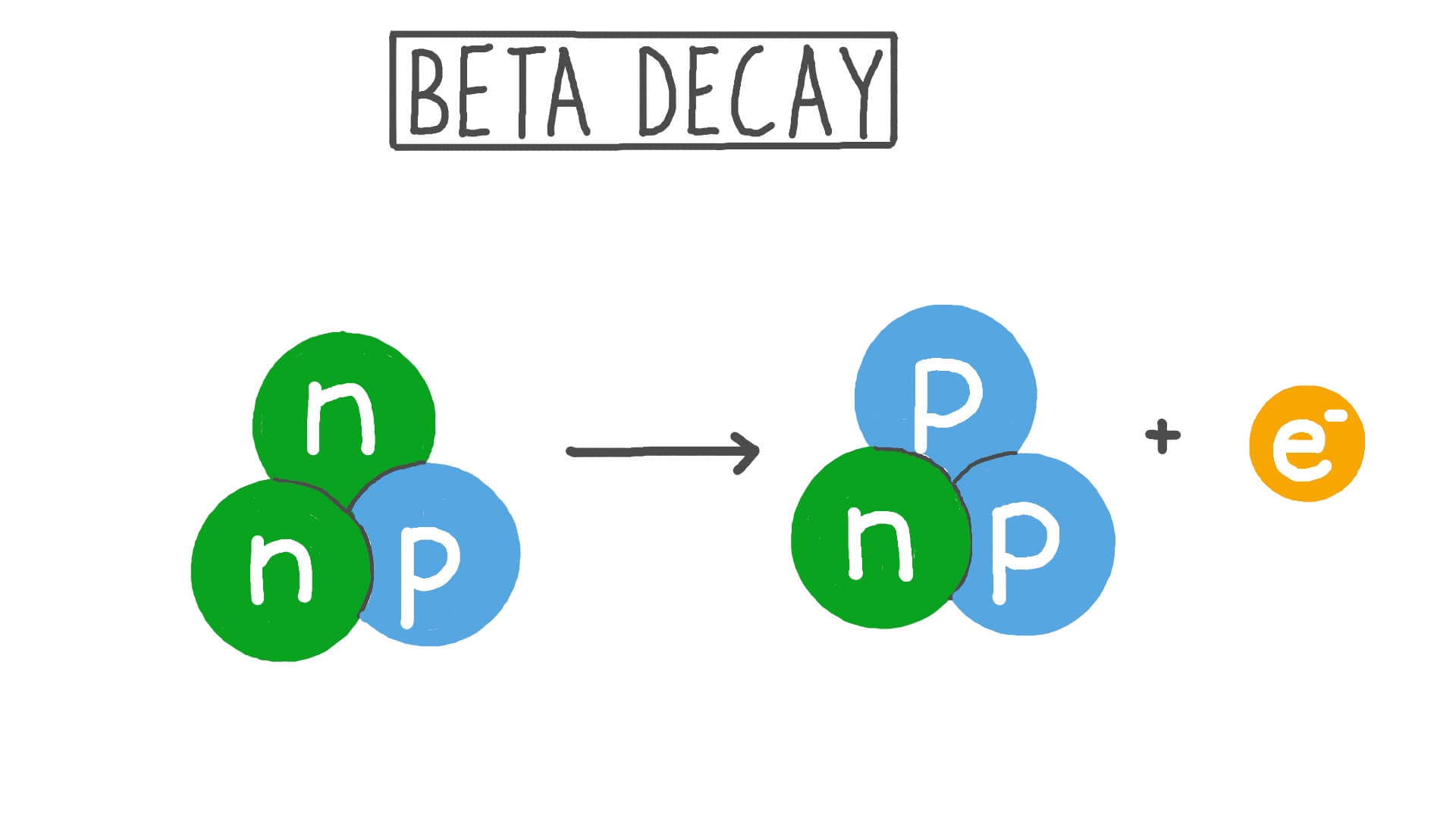
The term given to 'loose' electrons that come from radioactive decay
What is the other name for group 18th elements?A.Noble gases,B.Alkali metals C.Alkali earth metals,D.Halogens
A.Noble gases
which of the following atoms contains only three valence electrons:Li,B,N,F,Ne?
B
What is the electron configuration of radium?
[Rn]7s2
what is the differences between physical changes and chemical changes?
physical changes alter physical properties, while chemical changes transform substances into new substances