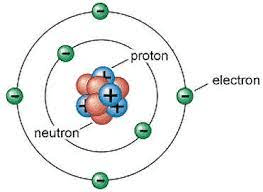
It has a negative charge.
electron
The oldest and simplest model
"billiard ball" (a solid ball)
These take part in bonding.
outer electrons
How many more electrons does it need?
two
An atom with a non-zero net charge
ion
It has a positive charge.
proton
The most accurate model
electron cloud
These are stronger than single or double bonds.
triple bonds
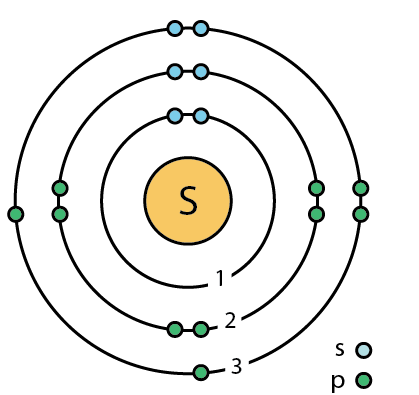
 How many electrons will it give?
How many electrons will it give?
two
Repeating at intervals
periodic
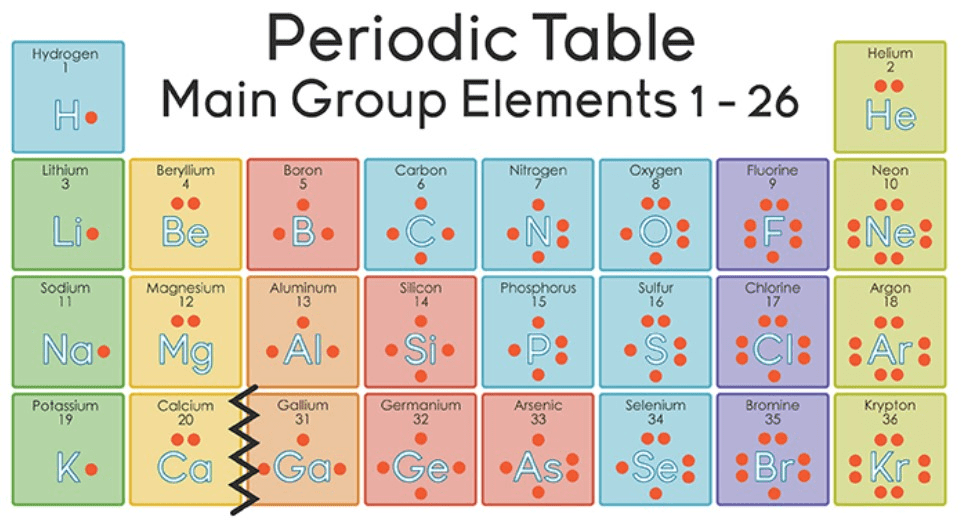
The atomic number will tell you how many there are.
protons
Inaccurate and not used anymore
Plum Pudding model
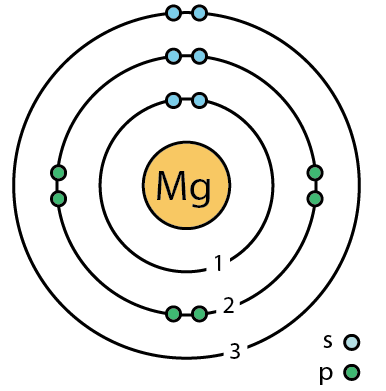
Stable?
No!
Which is probably the most stable?
MgCl
MgCl2
Mg2Cl
Here are dot models to help you:
MgCl2
(Magnesium can give two electrons, which will fill the outer energy levels of two Chlorine atoms)
What the dots represent:

outer electrons
A "packet" of light energy.
photon
Alpha particle experiments led to this model that is mostly empty space
Rutherford model
O = O
How many electrons are being shared?
four
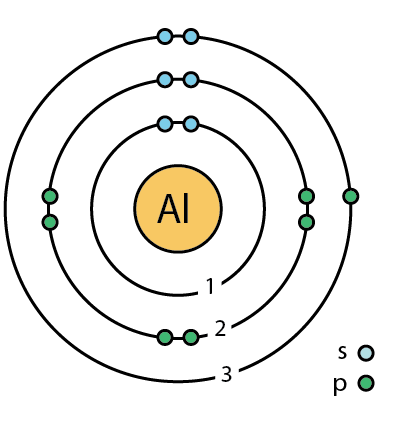
Which is most likely?
AlF
AlF2
AlF3
Here are dot models to help you:

AlF3
This is a naturally-occurring mineral.
These elements don't react with anything
Noble gases (last column of the periodic table)
It has no charge.
neutron
Gave Bohr the idea of energy levels
emission spectra
How many bonds would it form?
four
Quartz (the main mineral component in sand) is made of silicon and oxygen. What formula does it probably have?
SiO
Si2O
SiO2
Here are dot models to help you:

SiO2
Each silicon needs four additional electrons to be stable.
Each oxygen needs two additional electrons to be stable.
electronegativity