What is the definition of a mole?
A measure of amount equalling 6.022*1023 objects.
What is the conversion factor necessary to convert from volume of gas to moles of gas at STP? Give both the value and units of the conversion factor.
22.4 L of gas/mole
In the following balanced equation, what is the ratio of N2(g) to NH3(g)?
N2(g) + 3H2(g) --> 2NH3(g)
1 N2 : 2 NH3
A reaction that is exothermic has a ∆H that is:
Negative, or less than 0
In the relationship
(P_1)/(T_1)=(P_2)/(T_2)
The units of temperature must be in:
Kelvin
The empirical formula of the compound with formula C8H10 is
C4H5
A student is trying to find the number of moles in 9.34g of sodium sulfate. What value would they need solve this?
What is the formula for percent yield?
(actual\ yield)/(t\he\o\r\e\t\i\c\a\l\ yield) *100%
Decomposition reactions are often endothermic because:
They involve the breaking of bonds, which requires energy
In the following diagram of a manometer, the pressure of the gas sample is higher or lower than atmospheric pressure?
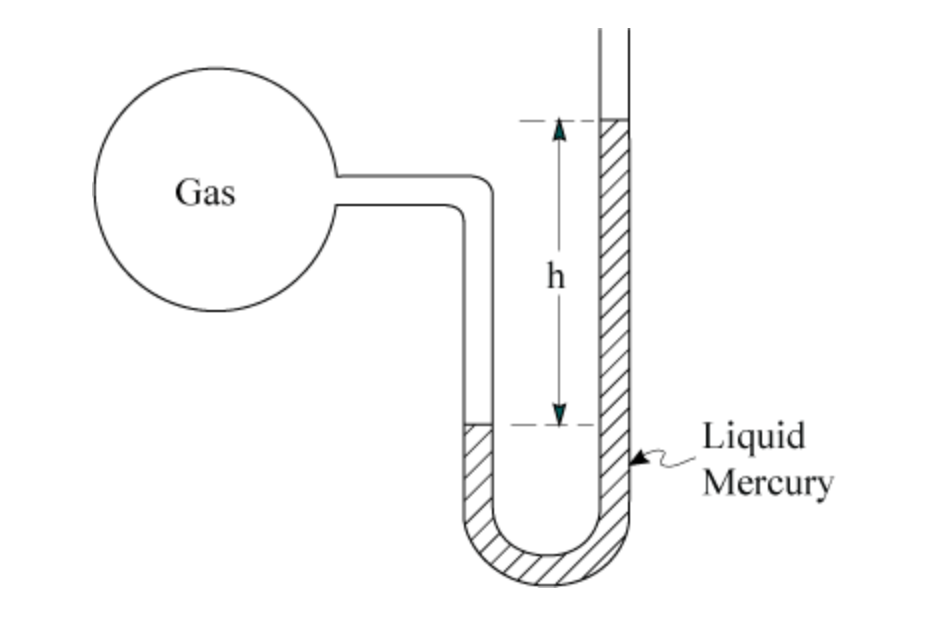
Higher
1 mole of solute per liter of solution
How many hydrogen atoms are contained in 1.00moles of methane gas?
2.409*1024 atoms of hydrogen
The reactant that determines the amount of product that is ultimately formed is known as the
Limiting reactant
A substance that tends to resist temperature change is one with a high or low specific heat?
High, because it means more energy is needed to change the temperature
If the pressure on a sealed sample of gas is increased but its temperature stays constant, its volume must
Decrease
What is the percent composition by mass of nitrogen in nitrogen dioxide? (Give answer to 3 sig figs)
30.4% Nitrogen by mass
Write the setup to solve for the moles of sodium chloride in 150.0mL of 0.250 M sodium chloride solution
150.0mL*(1L)/(1000mL)*(0.250mol)/(1L)
If 2.50 moles of lithium are reacted, how many moles of hydrogen gas will be formed?
2Li(s) + 2H2O(l) --> 2LiOH(aq) + H2(g)
1.25 moles of hydrogen gas
Consider the following thermochemical equation:
2H2(g) + O2(g) --> 2H2O(g) ∆H = -571.6kJ/mol
How much heat is produced for every ONE mole of H2 reacted?
-285.8 of heat are released per mole of H2 reacted
According to kinetic molecular theory, the collisions between molecules of a gas are:
What volume ammonia gas at STP must be dissolved to make 0.500 liters of 0.65M ammonia solution.
7.3L of ammonia gas
If a compound has an empirical formula of C1H1 and a molar mass of 26.04g/mol, give its molecular formula.
C2H2
How many moles of diphosphorous pentoxide will be formed from the reaction of 8.00moles of phosphorous with 9.00 moles of oxygen gas?
4P(s) + 5O2(g) --> 2P2O5(s)
3.60 moles of phosphorous pentoxide
Calculate the specific heat of 50.00g substance that warms up 10.0°C when heated with 200.0 J of heat.
0.400 J/(g°C)
What is the volume of 0.30moles of nitrogen gas kept at 2.56atm and 24.6°C?
2.9L of nitrogen gas