The key difference between covalent and ionic bonding
What is ionic bonding has a donator and acceptor of electrons whilst covalent bonding shares the electrons
The two main types of double displacement reactions
What is neutralisation and precipitation
The definition of a periodic trend
What is a a pattern in an element's properties, such as atomic radius or electronegativity, that appears predictably across the periodic table
The three conditions necessary for a reaction to occur according to collision theory
What is: A collision must occur, with enough energy, and correct orientation
This term describes the energy required to remove an electron from an atom, and it generally increases across a period due to this atomic trend.
What is ionisation energy
The name of the polyatomic ion with the equation of: SO42-
What is sulfate
The more reactive metal out of Gold and Copper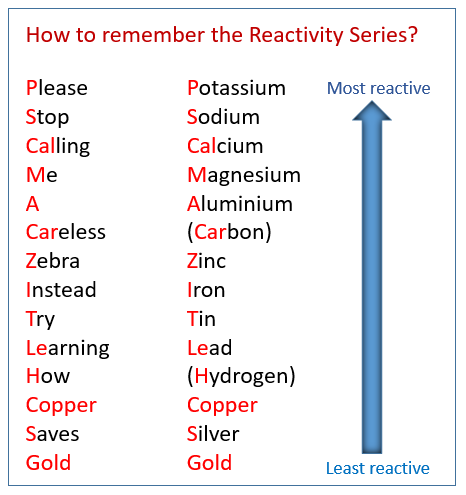
What is copper
The trend related to atomic radii
What is increase to the left and down.
The factor affecting a rate of reaction ascociated with Kinetic energy
What is temperature
The electron configuration of Lithium
2,1
The chemical name of N2O3. (Covalent)
What is Dinitrogen Trioxide
The two products formed under a neutralisation reaction.
What is a salt and water
The periodic trend in Nonmetalic Trends
What is increases to the right and up
The factor affecting a rate of reaction ascociated with collision sites
What is surface area
This experiment demonstrates the existence of shells within an atom.
What is the flame test
The chemical name of AgNO3
What is Silver Nitrate
The salt formed between Hydrogen Iodide (HI) and Sodium Hydroxide (NaOH)
What is Sodium Iodide (NaI)
The predictable ionic charge of group 14 (IV)
What is +/- 4
The factor affecting a rate of reaction ascociated with altering the amount of possible collisions to occur in relation to molecules.
What is concentration.
The electron configuration of Chlorine
What is 2, 8, 7
The balanced chemical formula of Copper Phoshpate
What is Cu3(PO4)2
The product that forms a precipitate between Silver Nitrate and Sodium Bromide
What is Silver Bromide
The creator of the modern periodic table
Who is Dmitri Mendeleev
The biological factor that alters the speed at which the biochemical reaction occurs
What is an enzyme
The overall charge of an electron
What is negative