Describe what an ionic bond is. [1]
[1] (the electrostatic force of) attraction between oppositely charged ions
Describe what a covalent bond is. [1]
[1] (electrostatic force of) attraction between shared pairs of e- and the nuclei (of two atoms)
Describe what VSEPR theory is and how its used to predict molecule shapes. [2]
States that electron pairs (bonding and lone pairs) repel
arrange to minimise repulsion // get as much distance away as possible
Explain which property of graphene allow sit to be used for...
B. body armour
B. Body armour
Graphene is very strong and lightweight, with a high tensile strength due to its strong covalent bonds between carbon atoms, making it suitable for body armour.
Describe what a metallic bond is. [1]
[1] (the electrostatic force of) attraction between delocalised electrons and positive metal ions
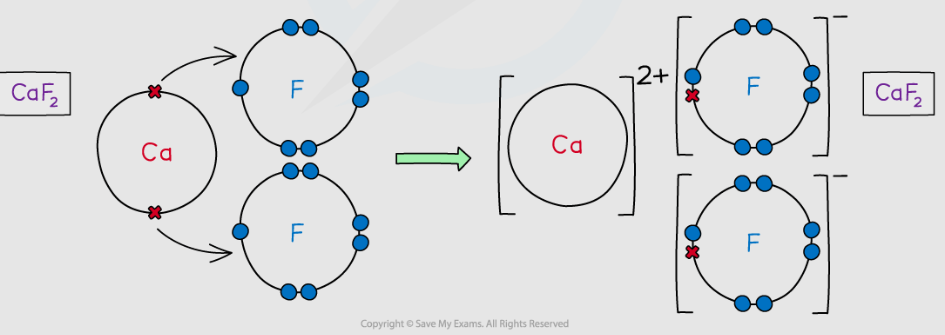
Draw a dot-cross diagram for calcium fluoride. [2]
Draw a dot-cross diagram for SF4
State the shape and bond angle for the S-F bonds
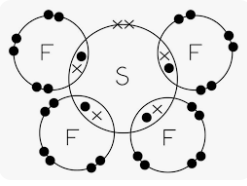
(See saw, 90 and 120)
What are the bond angles for the following molecules:
A. BeCl2
B. BCl3
C. CH4
A. 180
B. 120
C. 109.5
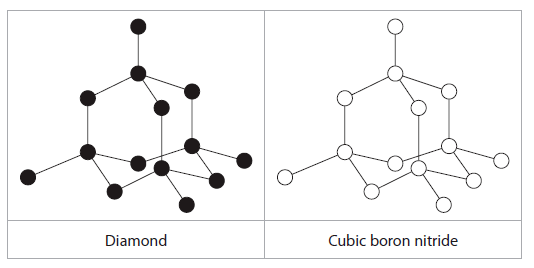
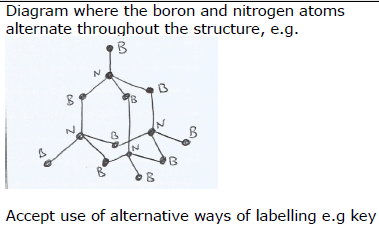
(b) The structure of the cubic boron nitride corresponds to the diamond structure. The boron and nitrogen atoms alternate throughout the structure.
(i) In the left hand box, the diagram shows a section of the diamond structure, where each black circle represents a carbon atom.
In the right hand box label all the nitrogen and boron atoms in the diagram of cubic boron nitride. (1)
ii. Label the bond angle of for diamond
Explain why pure iron is not used for constructing bridges and instead steel is used. [3]
[1] pure iron is softer and not as strong as steel
[1] because steel has carbon atoms added to its structure
[1]which disrupts the layers and doesn't allow them to slide easily over each other
Put the following ions in order of decreasing ionic radius.
Cl-, S2-, P3-
biggest P3- > S2 > Cl- smallest
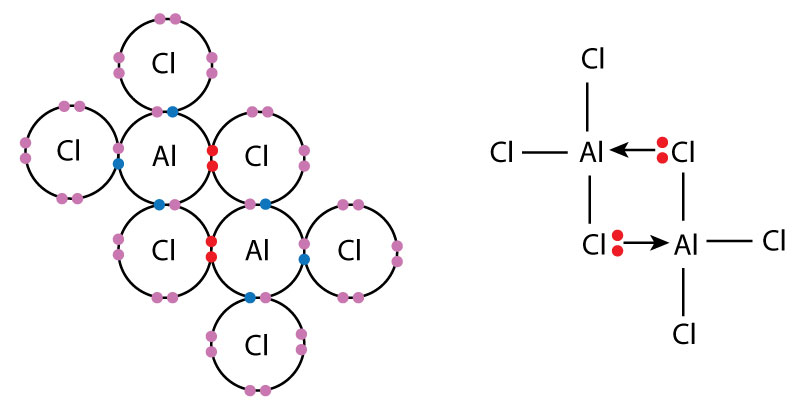
Draw the dimer forming for Al2Cl6.
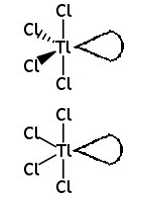
Thallium also forms ions containing chlorine, for example the TlCl43− ion.
In this ion, the thallium atom has 10 electrons in its outermost shell.
Phosphorus in phosphorus pentachloride, PCl5, also has 10 electrons in its outer shell.
Draw the shape of the TlCl43− ion and predict the bond angles.
Include any lone pairs of electrons that influence the shape.
[1] diagram
[1] estimated bond angle of 120 or less between equatorial Cls
[1] at least one estimated bond angle of 90 or less between axial and equatorial Cl
Explain two ways in which the physical properties of diamond and graphite differ.
Refer to their structure and bonding in your answer.
(4)
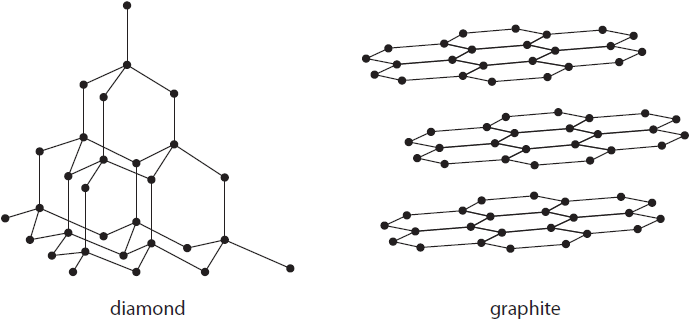
[1] diamond is hard and graphite is soft
[1] b/c diamond has rigid lattice / weak forces between layers in graphite
[1] graphite conducts electricity and diamond does not
[1] because graphite has delocalised e- (free to move) / diamond does not
Explain why metals are good conductors of heat considering their bonding and structure. [2]
[1] have delocalised e-
[1] when heated e- move faster and are able to transfer heat quicker // atoms vibrate faster
Complete the following statements.
Which would be the most polarising?
______ charge and ______ size
Which would has the most polarising power?
______ charge and ______ size
More polarizable anion if…
High charge of anion and Large size anion
Polarisation power increases with
High charge of cation and small size cation
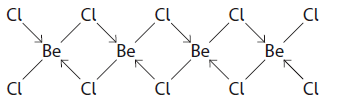
Describe how the two types of covalent bonds form for this dimer of BeCl2 [2]
[1] a line/covalent bond is when electrons come from both atoms
[1] arrow/dative/co-oordinate bond which is (a lone pair of) e- donated from chlorine / only one atom
Justify the shape of XeF4 and include the bond angle observed. (4)
[1] 6 e- pairs around central atom
[1] States electron pairs arrange to minimise repulsion
[1] square planar shape (due to 2 lone pairs)
[1] bond angles of 90 degrees
Explain how the structure and bonding in graphene makes it useful for touchscreens. [3]
[1] graphene is a layer of hexagons of carbons with 3 bonds per carbon
[1] with one delocalised electron per carbon atom that is free to move
[1] allowing for conduction of electricity
[1] 1 atom layer makes it useful for thin screens
Draw a labelled 2D diagram to show the metallic structure of Aluminum
Explain the difference in bonding between sodium chloride and aluminum chloride, using the electronegativity values show. (3)
Electronegativity values:
Sodium (Na): 0.9
Aluminium (Al): 1.5
Chlorine (Cl): 3.0
[1] Al is more electronegative than Na
[1] difference in EN is greater between Na-Cl than Al-Cl
[1] AlCl₃ shows more covalent character (smaller difference / polarisation of Cl⁻).
Compare and contrast the covalent bonding in Ethane vs Ethene in terms of orbital overlap. (4)
[1] Both have sigma bonds
[1] s orbital end on overlaps
[1] Ethene has a pi bond
[1]p orbital sideways overlap
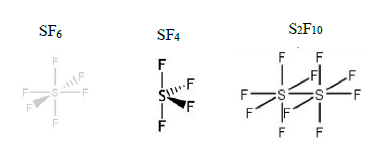
Explain is SF4 more reactive than SF6 considering the shape and bonding? [1]
Suggest why is S2F10 more reactive than SF6 using the idea of bond strength OR bond length between S-S and S-F. [2]
[1] S-S bond (in S2F10) is weaker than the S-F bond (SF6) // S-S bond is shorter than the S-F bond(SF6)
[1] SF bond has (six) strong S-F bonds to break
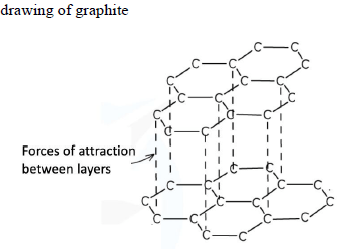
Draw a labelled 3D structure of graphite, showing two layers of at least 13 carbon atoms each and the forces between the layers. [2]
[1] at least two layers of hexagons w/ at least 3 hexagons per layer
[1] labelled forces of attraction between layers
Explain why the melting point of aluminium is much higher than the melting point of sodium. [3]
[1] Aluminium ions have a bigger positive charge (3⁺) than sodium ions (1⁺).
[1] Aluminium atoms are smaller in size, so the delocalised electrons are closer to the nuclei.
[1] Aluminium also provides more delocalised electrons per atom.
These factors all result in a stronger metallic bond, so aluminium has a much higher melting point.