What is mass?
It is the amount of "stuff" in an object
positive and negative ions are called
cations and anions
In the periodic table, elements in the vertical columns form this
Groups
What is a polymer?
a long chain made up of repeating units called monomers
A hydrocarbon with only carbon-carbon single bonds
alkanes
What are the 4 most common atoms in organic molecules contain?
carbon, hydrogen, oxygen, nitrogen
What is the charge on all group 1 metal ions?
+1
what is a chemical bond?
the force of attraction between two atoms
6
What is matter?
something that has mass and volume
Metals react to..
Non-metals react to...
gain electrons
The horizontal rows on the periodic table are called
Periods
Name 3 types of polymer which we learned about
polystyrene, polyethene, polyvinylchloride, polytetrafluoroethane
Name the hydrocarbons with carbon-carbon double bonds and triple bonds
alkenes, alkynes
What is the alcohol functional group?
O-H
What is the charge of all Group 16 ions?
2-
all atoms react to do what?
gain a full valence shell of electrons
what does the mass number of an atom tell you?
the number of protons + neutrons
The smallest unit of an element is a what?
An atom
what is the outermost electron shell called?
the valence shell
The elements in group 18 are known as the--
Noble Gas
What is the process that makes polymers called?
polymerisation
What are the products formed from complete and incomplete combustion?
water and carbon dioxide
water and carbon OR carbon monoxide
If a molecule has a -COOH group, what type of molecule is it?
a carboxylic acid
For an ionic bond to form...
electrons must be given by the metal to the non-metal
Metallic, ionic, covalent
what does LDPE stand for?
low density polyethylene (polyethene)
What is an element?
A pure substance that cannot be broken down.
What rule is used to work out electron configuration?
the 2,8,8,2 rule
Elements that are good conductors of electricity are called
metals
What does PTFE stand for?
polytetrafluoroethane
Name the first 6 alkanes
methane, ethane, propane, butane, pentane, hexane
name this molecule
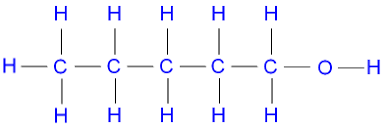
pentanol
If the charge on sodium is +1 and the charge on oxygen is 2- what is the formula of the compound sodium oxide?
Na2O
What type of bonding do non-metal atoms form between themselves?
covalent
what is the electronic configuration of sodium? (11 electrons)
2,8,1.
How many elements are in the modern periodic table?
118
what is an electron shell
a 3D area of space orbiting the nucleus where you are most likely to find electrons
Why is the periodic table helpful to understanding chemistry?
It organises the elements into order of their atomic number and shows repeating trends/patterns in the elements
What is the relationship between plastics and polymers?
All plastics are polymers, but not all polymers are plastics
Where do most hydrocarbons which we use come from and how do we get them from this substance?
crude oil, fractional distillation
Name this molecule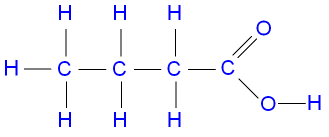
butanoic acid
what is the formula of a compound formed from calcium (2+) and nitrate (NO3-)
Ca(NO3)2
Describe the three types of bonding
metallic - cations of metal with free moving electrons flowing around them
ionic - atoms transfer electrons to form ions, electrostatic force of attraction between these ions
covalent - atoms share electrons
What is bioaccumulation?
Bioaccumulation is the build up of microplastics or other chemicals in the body of apex predators due to them eating lots of smaller organisms with the chemical in their bodies.