Give 3 lab safety rules.
Tie your hair back
Wear safety goggles
Follow instructions
Read safety symbols
Give the definition for a control variable
Something that stays the same in an experiment
The scientific unit for energy is ______. The symbol is ____.
Joule; J
Which method can be used to separate an INSOLUBLE solid from a liquid?
A: crystallization/evaporation
B: filtration
C: chromatography
D: distillation
B: filtration
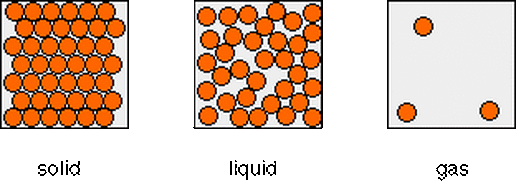
Give the three states materials can be in
Solids, liquids, gases
Name the two types of flames on a bunsen burner.
Roaring flame, safety flame
Give the definition for independent and dependent variable.
Independent variable - what you change
Dependent variable - what you measure
Power is the... The unit is ... and the symbol is...
Power is the rate at which (how fast) energy is used. The unit is Watt and the symbol is W.
Evaporation/crystallization
Draw and label a particle model for solids, liquids, and gases.
What is this hazard symbol called?

Flammable
You are measuring temperature change with the use of different insulators. Identify the independent and dependent variable.
Independent variable - different insulators
Dependent variable - temperature change
A 2 kW electric fire is used for 3 hours. Calculate the energy used in kWh.
Use the formula:
Energy (kWh) = Power (kW) x time in hours
You must use UFIFA for points.
U: kWh
F: Energy = Power x time in hours
I: Energy = 2 kW x 3 hours
F: ---
A: Energy = 6 kWh
Give the difference between simple and fractional distillation.
Simple distillation: separating solvent from solution when the boiling points are very different
Fractional distillation: separating solvent from solution when the boiling points are close to each other (e.g., ethanol and water)
How does increasing temperature affect gas pressure?
Increasing the temperature makes gas pressure go up. The particles move faster and collide with each other and the walls of a container more, so they have more energy.
What is this hazard symbol?

Corrosive
Dropping a ball from different heights could affect how high it bounces. Identify the dependent and independent variable.
Dependent - how high the ball bounces
Independent - different heights the ball is dropped from
Gas central heating is used for two weeks, using 320 kWh of energy. If 1 kWh of gas costs 5p, calculate the cost of using the central heating.
Use the formula:
Cost = energy used in kWh × cost of 1 kWh
You must use UFIFA for points.
U: pounds
F: Cost = energy used in kWh x cost of 1kWh
I: Cost = 320 kWh x 5 p
F: ---
A: Energy = 1600 p = 16.00 pounds
Describe the process of simple distillation. You may use pictures and/or words.

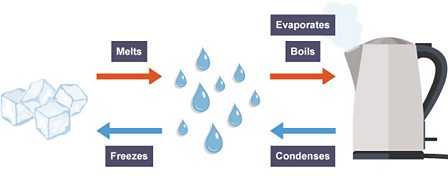
Draw and label a diagram to show how ice changes state to become water and then steam. You must include the name of the change of state (e.g., melting)
Give the difference between a safety flame and a roaring flame.
Roaring flame - blue, very hot, more oxygen comes through air hole in collar
Safety flame - yellow, easy to see, not as hot
Reaction times can be investigated by dropping a ruler and seeing how quickly someone can grab it. Identify the dependent and independent variable.
Dependent - How quickly someone caught the ruler.
Independent - the person catching the ruler
A 3 kW water heater is used for 1 hour. If 1 kWh costs 16p, calculate the total cost of using the water heater.
Use the formulas:
Energy (kWh) = Power (kW) x time in hours
Cost = energy used in kWh × cost of 1 kWh
You must use UFIFA for points.
Part 1: Calculate energy
U: kWh
F: Energy = Power x time in hours
I: Energy = 3kW x 1 hour
F: ---
A: Energy = 3kWh
Part 2: Calculate Cost
U: pounds
F: Cost = energy used in kWh x cost of 1 kWh
I: Cost = 3kWh x 16p
F: ---
A: Cost = 48 p
Answer the following two questions about chromatography correctly for 500 points.
1. Why is the line drawn in pencil on chromatography paper?
2. In the chromatogram below, what is the mixture on the left made of?

1. So the starting line doesn't bleed when it is in the solvent.
2. A, B, and C
Look at the picture below. Explain what happens to gas pressure when the volume of the ball is decreased (the ball is squashed to become smaller).

The gas pressure increases because when the volume is decreased (the ball is squashed), the gas particles collide with each other and the walls of the container more.