What are the three subatomic particles that make up atoms?
Protons, Neutrons, Electrons
What is an element?
A substance made up of only one type of atom
What side of the reaction will I find the reactants?
Left side
A chemical reaction that gets colder is an?
Endothermic Reaction
What pH range do bases have?
Greater than 7
What is the charge of protons, neutrons and electrons
Proton = positive
Electron = negative
Neutron = neutral
What is a compound?
A substance with 2 or more atoms chemically bonded together
State the two types of changes that can occur during a reaction and state if they can be reversed. Provide an example of each.
Physical change - reversible. Example, Ice melting into water and Salt dissolving in water
Chemical change - usually irreversible. Example, burning wood and rusting of iron.
The type of reaction that causes the temperature to increase.
What is exothermic?
What pH range do acids have?
0-6
Where do we find electrons in an atom?
In electron shells surrounding the atom
What are the three types of bonds?
Covalent, Ionic and Metallic
The form of a chemical reaction?
Reactants -> products
In an exothermic reaction, does the temperature of the surroundings increase or decrease? Why?
It increases because energy is released into the surroundings.
What pH does water have?
7
What is the name for the outermost electrons of an atom?
Valence electrons
What is the atomic mass made up of?
Protons, neutrons and electrons
Define the Law of Conservation of Mass
The law of conservation of mass states that mass cannot be created or destroyed in a closed system.
What is required for a combustion reaction to occur? What type of chemical reaction occurs?
Fuel, oxygen and heat (ignition source). Exothermic
What does pH stand for?
Power of Hydrogen
What sub-atomic particles make up the nucleus?
Protons and neutrons
What does the atomic number tell you?
The number of protons in the nucleus
What is the collision theory?
Particles must collide with enough energy and in the correct orientation to react. Increasing the frequency and energy of collisions increases the rate of reaction.
What happens to the temperature of the surroundings during an exothermic reaction?
During an exothermic reaction, the temperature of the surroundings increases because energy is released from the reaction into the environment as heat.
What is the name of the reaction between an acid and a base?
Neutralisation.
Fror the first 20 elements, what is the electron configuration for bohr diagrams?
2,8,8,18
In the compound below, how many atoms of each element are there?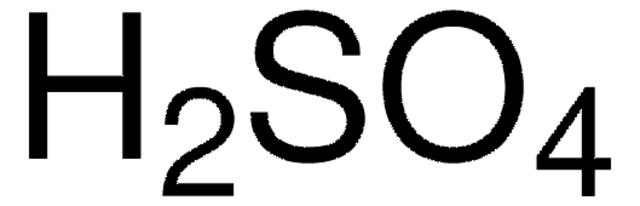
2 hydrogen (H), 1 sulfur (S), 4 oxygen (O)
Name the five factors that can increase the rate of a chemical reaction.
Temperature, concentration, surface area, pressure, and catalysts.
What is the key difference between complete and incomplete combustion?
The key difference is the amount of oxygen available:
complete combustion occurs with a sufficient oxygen supply, producing only carbon dioxide and water
Incomplete combustion occurs with insufficient oxygen, producing carbon monoxide, carbon, and water.
Explain why acids have a low pH and bases have a high pH.
Acids have a high concentration of H⁺ ions, which lower the pH. Bases have a high concentration of OH⁻ ions, which raise the pH.