What are the 4 states of matter?
Solid, Liquid, Gas, Plasma
Who first proposed the particle model of matter?
John Dalton
What is Viscosity?
The thickness of a liquid
What is kinetic energy?
Movement energy
Who first came up with the idea of atoms?
Democritus
melting
When particles are lined up in a regular arrangement and held close together, they are in what state of matter?
Solid
What are the two types of strength?
Tensile and compressional
If we add heat to a substance, what are we adding?
Kinetic energy.
All matter has what two things?
When a gas changes to a liquid, it is called . . .
condensation
When particles are far apart and move quickly on their own, they are in what state of matter?
Gas
What is density?
The amount of mass per volume
What happens to particles when we add heat?
They move faster as they have more kinetic energy.
What is diffusion? Give an example.
Tea bag in water, perfume, etc.
What are the processes by which a liquid turns to a solid, or to a gas?
solidification (freezing), and evaporation
Draw the particles in all 3 states of matter according to the Particle Model
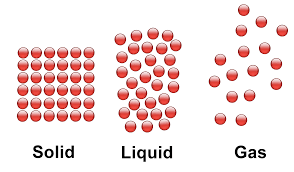
A very hard substance will ____________ easily, and is called ______________.
Break; brittle.
Heat causes substances to _________. When their temperature decreases, the substance ____________.
Expand; contract
Explain the difference between tensile strength and compressional strength
Tensile strength is a measure of the flexibility of the bonds between particles. Compressional strength is the ability to withstand large forces.
What is the process where a solid turns directly into a gas?
Sublimation
What is wrong with this representation of the Particle model?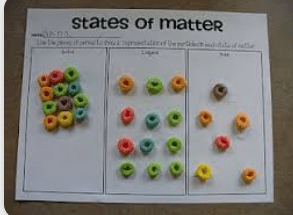
The liquid particles are shown with space between them, but liquids cannot be compressed because their is no space between the particles. They should also not be lined up in order as in liquids the particles move around each other.
What is compressibility? Which states of matter can be compressed and which cannot?
The ability of a substance to be squashed or compressed.
Gas can be compressed; Solids and liquids cannot be compressed.
What are expansion joints for?
To allow for substances to expand and contract with the addition and decrease of temperature
List 5 things we know about atoms
They cannot be divided
We cannot create or destroy them
Atoms have mass
They are too small to be seen
They are always moving
There are spaces between particles
Different atoms combine to form compounds
Etc