What state of matter does this represent - where the particles can move freely (have no fixed shape or volume)?

Gas
What happened to the hoop?

It cooled down, causing it to lose energy and the particles contracted (came closer together).
Is this a physical or chemical change?

Physical. Nothing new is being made, he is only changing the size of the wood.
A substance only made up of one type of particle is an
a) Element b) Compound c) Mixture

In diffusion particles move from an area of...
a) high concentration to low concentration
b) low concentration to high concentration
a) high concentration to low concentration

A substance goes from being a liquid to being a gas, what is this state change called?

Evaporation
Describe one way you could measure the volume of an object.
Volume of a box = width x height x length
Volume of an irregular object: Put it in a measuring cylinder so it is completely covered by water, measure the volume before and after putting it in. The difference is the volume of the object.

Which of these involves a chemical change and why?
a) Dissolving salt in water
b) Ice cream melting
c) Breaking an egg
d) Roasting a marshmallow
d) Roasting a marshmallow
Because something new is made, there is a colour change, a change in taste and smell, you cannot reverse it to uncook the marshmallow.

Is this an element, compound or mixture?

Mixture
Because there are different elements and compounds mixed together
Write an example of diffusion (particles moving from high concentration to low concentration until they are evenly spread out).

- Smell of baking filling a house
- Food colouring spreading throughout water
- The colour from skittles moving into the clear water
- Flavour from tea bag filling cup
- Perfume sprayed and filling the room
Which states have fluidity (can flow and be poured)?
Solids, liquids, and/or gases
Liquids and gases

Why are bridges made with gaps between each section?

So there is room for it to expand on hot days and not buckle, and so on cold days when it contracts it doesn't break.
Name any products in this equation:
Peter Parker + spider bite --> Spider-Man

What is the difference between a compound and a mixture?
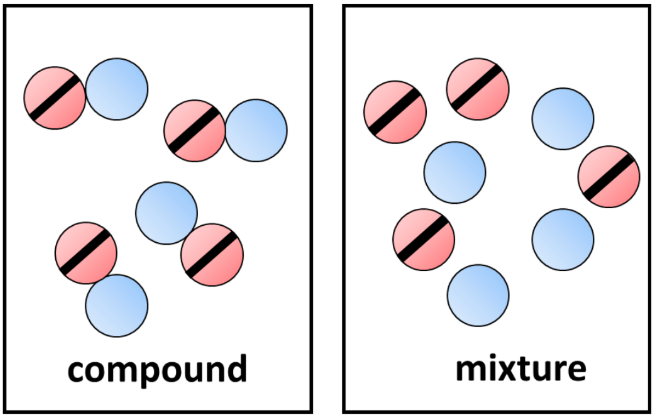
They both have more than one type of particle, but in a compound the different particles are bonded/joined together, in a mixture they are just mixed together, but don't bond.
Raro dissolves in water to make juice. What is the solute, solvent and solution?

Solute = raro powder
Solvent = water
Solution = juice
What is sublimation?
When something changes straight from being a solid to a gas

Why will a balloon pop if you keep pumping air into it? Use the word pressure in your answer.

Because the pressure on the inside of the balloon will become greater than the pressure outside the balloon, causing the balloon to break.
Write 3 signs that a chemical change has happened?

- Something new is made
- The reaction causes light or heat
- There is a change in smell or taste
- Fizzing or bubbling
- There is a colour change
- It cannot be easily reversed
How could you separate a mixture of salt and water?
Heat it up, until the water evaporates leaving the salt behind.

A solution is saturated when...
It cannot dissolve anymore of the solute (solid).

Why can a gas be compressed (made to fit in a smaller space/volume) and a solid can't?
Because the particles in gases are much further apart, so they can be forced closer together. The particles in solids are already tightly packed, so they can't be forced any closer.
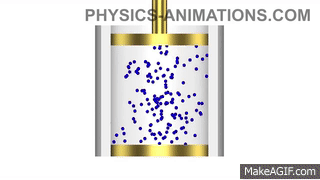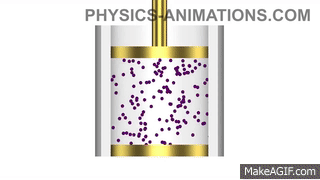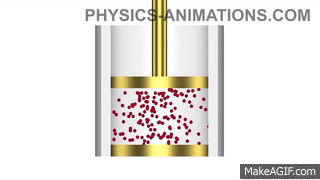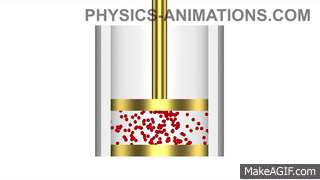
Water has a density of 1g/cm3. A piece of plastic has a mass of 24g and volume of 20cm3. Will the plastic sink or float in water? Show your working.
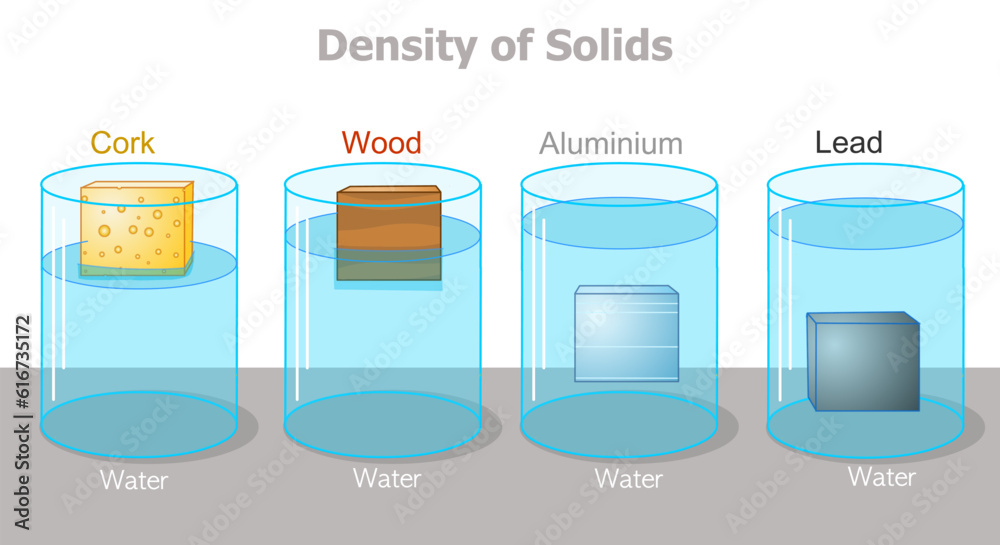
Density of plastic = mass ÷ volume
Density of plastic = 24 ÷ 20 = 1.2g/cm3
The plastic is denser than the water, so it will sink.
Write a word equation for when potato, oil and heat are used to make fries.

Potato + oil + heat --> fries
How could you separate a mixture of sand and iron filings?
With a magnet to draw out all the iron from the mixture, because it is magnetic, but sand is not.

Explain how the smell of your dank shoes can fill an entire room... use your understanding of diffusion to explain.

There is a high concentration of smelly odour particles in the shoe, they move to the rest of the room where there is a lower concentration of smelly particles. They continue to spread out until they are evenly spread throughout the room.
You open your door, dooming the rest of the household to smell your stink shoes.