What is an atom?
Small particles that make matter and all substances around us.
What is the periodic table for?
The periodic table helps us to understand their characteristics.
How many kinds of mixture do we have? What are the of this types names?
Homogeneous and Heterogeneous.
What is a molecule?
Two or more atoms of the same elements bonded together.
What are the elements of an atom?
Protons, electrons, neutrons
How many periods and groups in the periodic table?
7 periods and 8 groups
Why wine is homogeneous mixture?
Because the elements in wine combine to form a compound (an entirely new substance that can stand on its own), wine is a homogeneous substance.
What causes the molecule?
Molecules are the result of the interaction between atoms, to be more specific, electrons of the last shell.
What is nucleus of atom and what is inside it?
The nucleus is the positively charged centre of an atom and contains most of its mass. It is composed of protons, which have a positive charge, and neutrons, which have no charge.
What is the atomic number of an atom equal to?
The number of protons in the nucleus.
What are the differences between heterogenius and homogenius mixtures?
Unlike heterogeneous mixtures, homogeneous mixtures constitution is same. The composition of a homogeneous mixture is uniform and of heterogeneous is non-uniform.
What is chemical bond?
Molecules are formed thanks to the interaction between atoms
What is proton?
Elements that are located in group 8, what are they and what are the features of these elements?
The Noble gases. These are all gases, very stable in nature, do not react with other elements
Why chemical bonds are strong links formed between atoms?
They areresult of extremely string forces of attraction among the atoms.
If atoms contain charged particles, why do they not have a charge?
They contain equal numbers of positive protons and negative electrons.
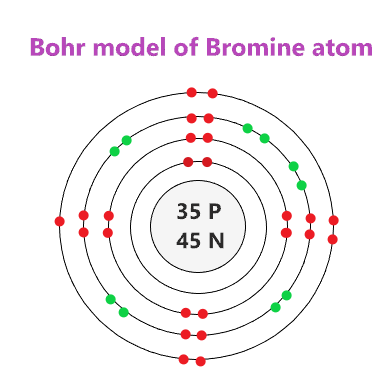
What period and group is this element in?
4th period and 7th group
Name 2 examples of homogeneous and 2 examples of heterogeneous mixtures. Explain why?
CO2 is a molecule or a compound? Explain why
Carbon dioxide is a chemical compound composed two oxygen atoms bonded to one carbon atom.