Name

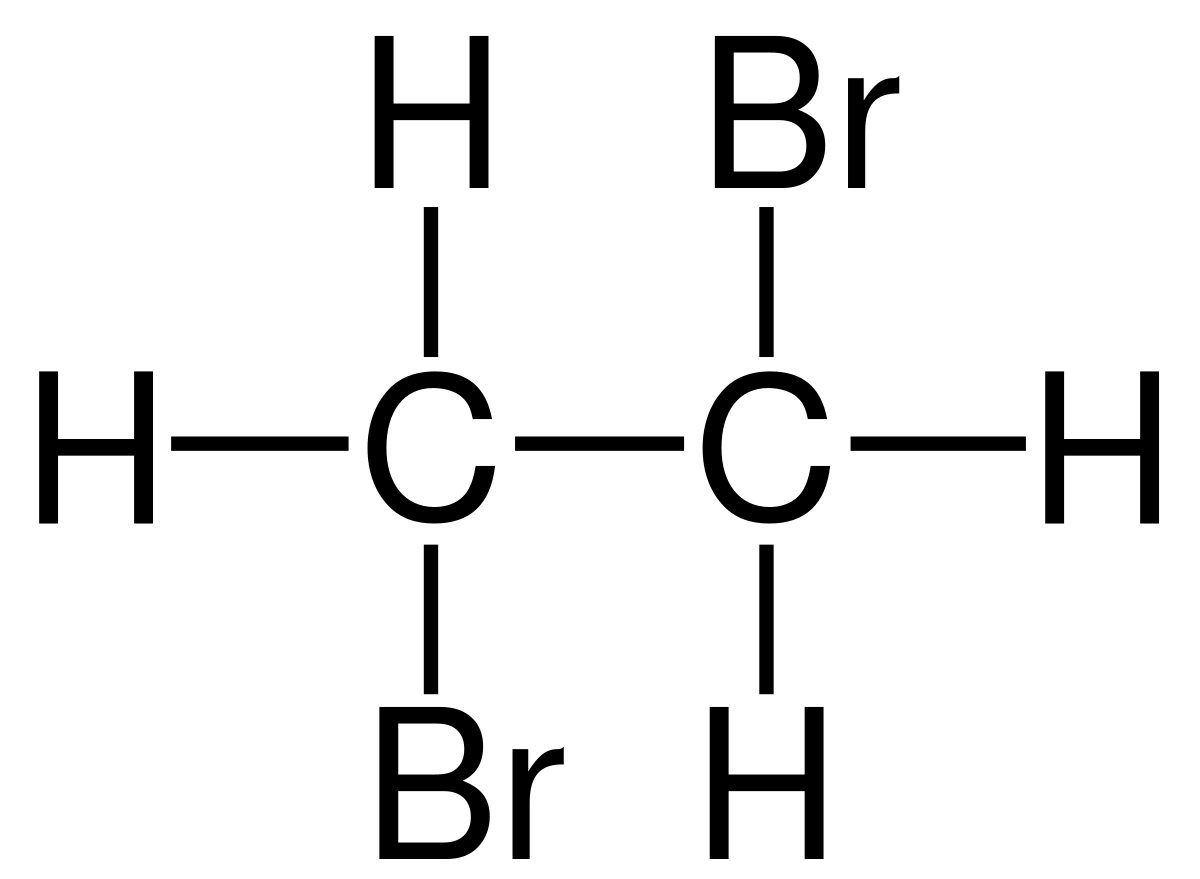
What is 2,3 dimethylpentane
What are the two products of COMPLETE combustion?
What is water and carbon dioxide?
Draw the product of ethene and Bromine (Br2)
What is the product formed between an alchohol and a carboxylic (alkanoic) acid?
An ester
Initiation, Propogration and....
What is the name of CH3CH2COOH
What is propanoic acid?
Carbon monoxide is produced
What is incomplete combustion?
Ethene + Water --> ???
What is ethanol?
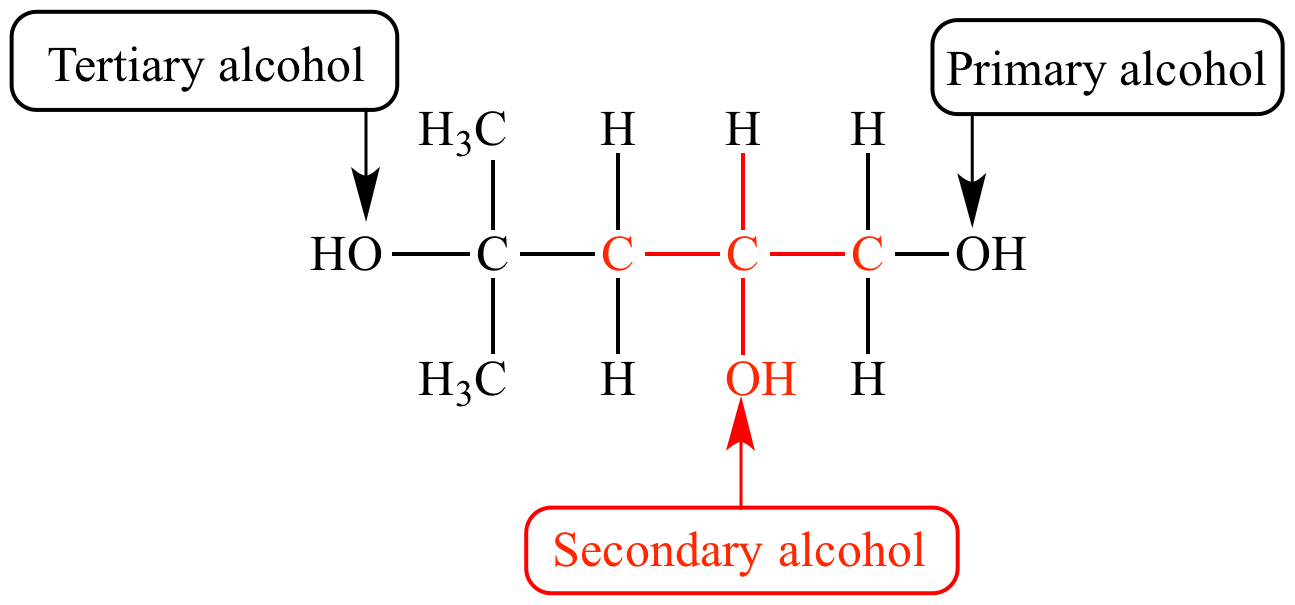
Name the alcohol

What is Propan-2-ol
 What does the dot represent?
What does the dot represent?
What is a radical?
Identify the functional group in CH3COOCH3
What is an ester?
What is the sum of the coefficients of this reaction and classify as complete or incomplete
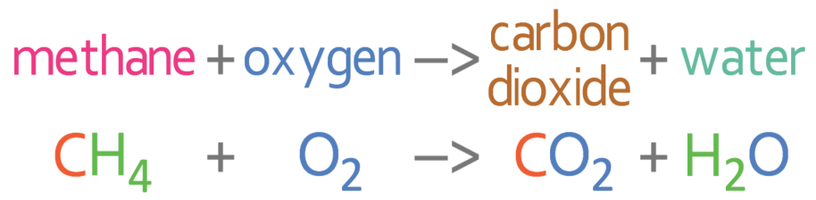
6 and complete
What are the reaction conditions required for the reaction of H2 to propene?
Nickel Catalyst, High Temperature and Pressure
The catalyst needed for an esterification reaction?
What is concentrated sulfuric acid?
How is Chlorine split during Initiation?
What is UV light?
Draw a secondary alcohol
Pentane is burned. A black substance is formed. What is the name of the substance and is this complete or incomplete combustion?
Carbon and Incomplete
Draw the chemical formed when ethanol and methanoic acid react in the presence of concentrated sulfuric acid
What is 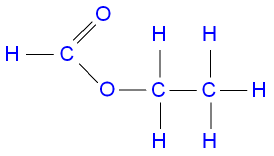
What is a methyl radical or hydrochloric acid (HCl)
What functional group is present 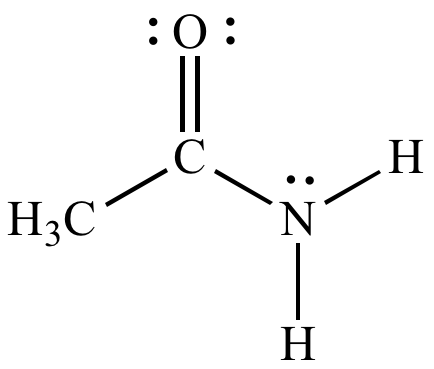
What is an amide?
Write and balance the complete combustion of ethane

Draw, using 3 propene monomer units, how propene forms polypropene?
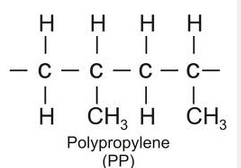
What does the secondary alcohol propan-2-ol produce when heated in the presence of the dichromate ion?
What is propanone?
A product of NaOH and Chloromethane
What is methanol (or sodium chloride)