Color
physical property
What is something that has mass and takes up space
matter
What are the two gas laws we discussed?
Boyles and Charles
protons are what charged?
positive
Made up of one type of atom
element
How many protons does Carbon (C) have?
6
Combustion
Chemical Property
What will the object do if it is less dense in water?
float
As pressure goes up volume goes down
Boyles
What two subatomic particles are located in the nucleus
protons and nucleus
Made up of two or more DIFFERENT atoms
compounds
What is the atomic mass of Sodium (Na)
23 amu
Solubility
Physical property
What is the formula for density?
D=m/v
As volume goes up temperature goes up as well
How many electrons can fit in the second energy level?
8
What are the two different type of mixtures
homogenous and heterogenous
How many neutrons does Gold (Au) have?
118
NaCl dissolves in water
physical change
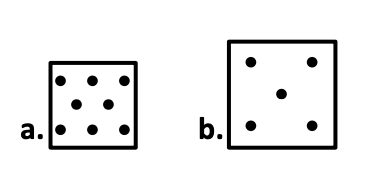 Which box is more dense?
Which box is more dense?
A
You accidentally left your basketball outside on a cold day. What happened to the volume of the ball?
decreased
What does the atomic number tell us?
how many protons and electrons
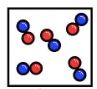 What is this diagram showing us
What is this diagram showing us
compounds; mixtures of compounds
How many electrons does Lead (Pb) have?
82
Food is digested
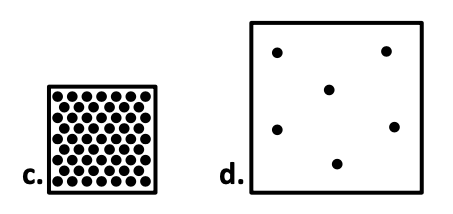
Which box is the most dense?
C
You shake a can of soda and then open it which decreases the pressure. What happens to the volume of the carbon dioxide in the soda?
increases
What you call an elements that have the same number of protons but different number of neutrons
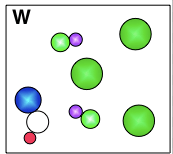 What is this diagram showing us a mixture of?
What is this diagram showing us a mixture of?
a mixture of compounds and elements
How many energy levels does Chlorine (Cl) have
3