1 mole of anything contains how many of that object?
6.02 E 23 of that item
Where do you find the molar mass of any element?
Periodic Table
What number do all of your percents have to add up to?
About 100%
Which chemical formula is always bigger, molecular or empirical?
Molecular Formula
Fill in the blank:
Twinkle Twinkle Little ________
Star
The water molecule has the formula of H2O. How many water molecules are in 1 mole of water?
6.02 E 23 molecules
Calculate the Molar Mass of NiS2
122.81 g/mol
What is the percent composition of ONLY oxygen in water, H2O?
88.9% O
If a chemical's molecular formula was Ga5F15, what would its empirical formula be?
GaF3
What is the name of this shape?

Trapezoid
Fill in the blanks
6.02 E 23 = _____ mole = ______ g of carbon
1st blank = 1 mole
2nd blank = 12 g
How much does 1 mole of sodium weigh?
23 g/mol
Determine the percent composition of every element in P2O5
P = 43.64%
O = 56.36%
What is the empirical formula for a compound that is 24.60% phosphorus and 75.4% fluorine?
PF5
What is the capital of China?
Beijing, China
Who discovered the mole number?
Avogadro's Number
Calculate the molar mass of (NH4)3PO4
149 g/mol
Determine the percent composition for every element in barium hydroxide, Ba(OH)2
Ba = 80.11%
O = 18.71%
H = 1.17%
Write both the molecular & empirical formula for the following chemical
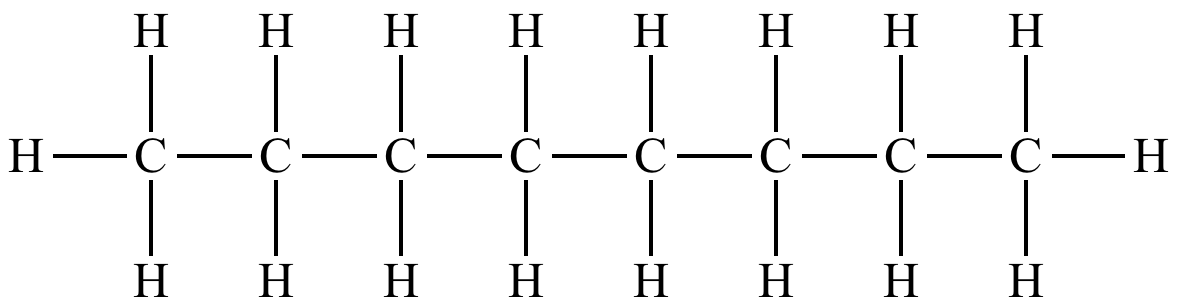
Molecular = C8H18
Empirical = C4H9
Find the next number in the sequence:
0, 1, 1, 2, 3, 5, 8, ___
13
How many moles are in 6.02 E 23 atoms of phosphorus?
1 mole
How much would 2 moles of methane, CH4, weigh?
32 g/mol
16 g CH4 = 1 mol
x2
32 g CH4 = 2 mol
Order the elements from greatest to least percent composition, C9H11NO3.
C = 59.66%
O = 26.49%
N = 7.73%
H = 6.12%
A compound is 23.81% arsenic and 76.19% bromine. If it's molar mass is 630 g/mol, what is its molecular formula?
As2Br6
What is the grammar mistake in the following sentence?
Its beginning to look a lot like Christmas.
It's