Which state of matter has a definite volume, but no definite shape?
Liquid
An atom of potassium (K) has how many protons?
19
In which nuclear process does an atom split into smaller atoms (used in nuclear reactors and bombs)?
Nuclear Fission
In which bond type do atoms share electrons?
covalent
Copy down the following chemcial equation and label the reactants and products:
2 Na + Cl2→ 2 NaCl
Reactants: Na and Cl2
Product: NaCl
A solution has a pH of 8.9. Is the solution acidic or basic.
Basic
When a substance is condensating, it is going from a ________________ to a ______________.
Gas to a liquid
How many valence electrons does an atom of Argon (Ar) have?
8
Which nuclear process occurs in the sun when 2 hydrogen atoms FUSE to create a hellium atom?
Nuclear Fussion
How many total atoms does the compound CuSO4 have?
6
A chemcial reaction that releases energy is know as a ___________ reaction.
Exergonic Reaction
In a solution of sugar and water, sugar is the (solute/solvent) and water is the (solute/solvent).
Sugar: Solute
Water: Solvent
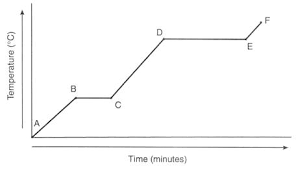
Look at the heating curve above.
Between which 2 letters is the substance melting?
Between B and C
How many electrons does a NEUTRAL atom of Bromine (Br) contain?
35
Rank the 3 types of radiation from least able to penatrate materials to most able to penatrate materials.
Alpha--> Beta--> Gamma
The compound N2 is (Polar/Nonpolar).
Nonpolar
A chemcial reaction that absorbs energy SPECIFICALLY in the form of heat is know as a ___________ reaction.
Endothermic Reaction
Decreasing temperature will (speed up/slow down) a GASEOUS solute dissolving into a LIQUID solvent.
speed up
In which state of matter change does a solid go directly to a gas?
Sublimation
What is the charge of an atom of calcium if it has 18 electrons? (Must include sign)
+2
Name one advantage of nuclear radiation.
No air pollution, more energy than fossil fuels
What is the name for the following covalent compound?
NO2
Nitrogen Dioxide
What kind of reaction is occuring in the chemical equation below?
2 H2O2--> 2 H2O+ O2
Decomposition Reaction
Look at the solubility curve above. If you add 120 g of KBr to a solution at 40oC, what type of solution will be created?
Supersaturated
Object A has a density of 0.6. It is placed in a liquid that has a density of 0.93. Will the object sink or float?
Float
What is the isotope name of an atom of carbon if it has 6 protons and 8 neutrons?
Carbon-14
If you start with 800 g of a substance, how much is left after 2 half lives?
200 g
What would the chemcial formula be for an Ionic compound containing Aluminum and Oxygen?
Al2O3
Write down the following chemcial equation, then balance it.
__ Li + __ H2O → __ LiOH + __ H2
2 Li + 2 H2O → 2 LiOH + 1 H2
Look at the solubility curve above. At what temperature would a saturated solution be formed if you added if you added 200 g of KNO3?
90oC