a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule
chemical formula
a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons and positively charged metal ions
metallic bond
what's the difference between physical and chemical property?
chemical property may only be observed by changing the chemical identity of a substance. physical property is observed or measured.
a reaction where two or more elements or compounds combine to form a single compound
combination reaction
property of a solid, liquid or gaseous chemical substance
solubility
a formula giving the proportions of the elements present in a compound but not the actual numbers or arrangement of atoms.
empirical formula
A covalent bond, also called a molecular bond, is a chemical bond that involves the sharing of electron pairs between atoms
covalent bonding
color, smell, freezing point, boiling point, melting point
physical property
a type of chemical reaction in which a single compound breaks down into two or more elements or new compounds
decomposition reaction
no more solute can be dissolved in the solvent.
saturated solution
a formula giving the number of atoms of each of these elements present in one molecule of a specific compound
molecular formula
Two hydrogen atoms will also come together to form a
single covalent bonding
Flammability.Enthalpy of formation. Heat of combustion. Oxidation states. Chemical stability.
chemical property
where an element reacts with a compound and takes the place of another element in that compound.
single replacement reaction
When a solution can have solute added and dissolved
unsaturated solutions
a formula unit is found
mole
where two pairs of electrons are shared between the atoms rather than just one pair.
double covalent bonding
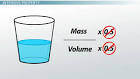
extensive property
a type of chemical reaction where two compounds react, and the positive ions and the negative ions of the two reactants switch places, forming two new compounds or products.
double replacement reaction
a solution that contains more of the dissolved material than could be dissolved by the solvent
supersaturated solution
formulas are used in what?
chemistry
a chemical bond between two atoms involving six bonding electrons instead of the usual two in a covalent single bond
triple covalent bonding
is a property of matter that depends only on the type of matter in a sample and not on the amount
intensive property
what's another name for double replacement reaction?
metathesis
mixture has the same uniform appearance and composition throughout
homogeneous