This is the most reactive group
Group 1 (1A)
This element has 12 protons
Magnesium
Periods are read this way (up and down OR side to side)
side to side (hint: period comes at the end of a sentence--and sentences are read side to side)
This is a positive subatomic particle
proton
What does the mnemonic APE mean
Atomic # = # of Protons = # of Electrons
This is the direction groups face (up and down OR side to side)
up and down
This element has an atomic mass of 12
Carbon
Periods tell us this
How many electron shells an element has
This is a NEGATIVE subatomic particle
electron
What does the mnemonic MAN help us remember?
Mass (rounded) - Atomic # = # of Neutrons
This is the group that DOES NOT react with other elements
Noble Gases, Group 18
This element has 4 electrons
Beryllium
This is the number of elements in period 3
8
This is a NEUTRAL subatomic particle
neutron
How many neutrons boron has
6
Groups tell us this these 3 things
Elements have similar properties, reactivity, AND SAME number of valence electrons
This element has 8 protons
Oxygen
This is the number of electron shells for all elements in period 4
4
This is another name for energy level
electron shell, electron cloud, electron orbital, e-cloud
How many valence electrons aluminum has
3
This is the group that has a full valence electron shell
Group 18 (8A)
This element has only 1 shell and 1 proton
Hydrogen
This element has the most mass of elements in the 2nd period
Neon
These are elctrons in the last, outer shell of an element
VALENCE electrons
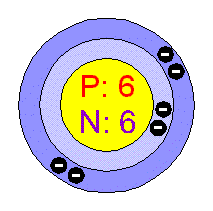
Bohr model for carbon