Where do you find the protons and neutrons?
nucleus

How many neutrons does Gold have?
118 Neutrons
Do neutrons have a positive or negative charge?
neither, they have a neutral
Point an arrow to where are the protons and neutrons are located
Who made the Plum Pudding model of an atom?
J.J Thomson
Valence electrons are located in:
A. Energy levels closest to the nucleus
B. All energy levels
C. Energy levels with the highest energy
C
How many electrons can the second shell hold up to?
8
Draw the Lewis dot diagram for Oxygen?
What is an isotope?
An isotope is any form of a chemical element that has an equal amount of protons but differ in the amount of neutrons.
How are isotopes different?
They have the same number of protons. The atomic number is decided by the number of protons. Isotopes have different mass numbers, though, because they have different numbers of neutrons.
When atoms share electrons it is called a...
Covalent Bond
What are the three main types of bonds?
Ionic
Covalent
Metallic
Why does electronegativity increase across a period in the periodic table?
As the number of protons in the nucleus increases, the electronegativityor attraction will increase. Therefore electronegativity increases from left to right in a row in the periodic table.
What is Electronegativity?
a measure of the tendency of an atom to attract a bonding pair of electrons.
How are ionic bonds formed?
Ionic bonds are formed between a cation, which is usually a metal, and an anion, which is usually a nonmetal.
What are the seven diatomic molecules?
hydrogen, nitrogen,oxygen, fluorine, chlorine, bromine, and iodine
Create a haiku so you can remember the seven diatomic molecules
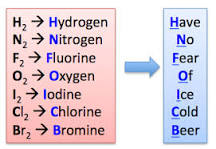
What unit increases as you go across the periodic table?
Atomic mass
What are the appropriate names for rows and columns on a periodic table?
periods
groups
What is the difference between Polar covalent and nonpolar covalent?
In covalent bonds two atoms share the electrons equally and in nonpolar covalent bonds they are unequal.
What differs between an ionic and covalent bond?
Ionic bonds are between a non-metal and metal and Covalent bonds are between 2 non-metals.
How many electrons are shared in a single bond?
2
True or False: Triple bonds share 8 electrons
False
Balance the following equation:
CH4 + Cl2 = CH2Cl + HCl
CH4 + 4Cl2 = CH2Cl + 4HCl
A very famous chemist was found murdered in his kitchen today. The police have narrowed it down to six suspects. They know it was two man job. Their names: Felice, Maxwell,Archibald, Nicolas, Jordan, and Xavier. A note was also found with the body:
26-3-58/28-27-57-16.
Who are the killers>
Felice and Nicolas- the numbers correspond to the atomic numbers on the periodic table
Fe-li-Ce/Ni-Co-La-S
