Rutherford’s Gold Foil experiment established which of the following features of the atom?
A. The atom is a solid sphere
B. The electron field has fixed electrons
C. The nucleus contains electrons and protons
D. The atom is mostly empty space but has a dense central nucleus
D. The atom is mostly empty space but has a dense central nucleus
What does this diagram represent?
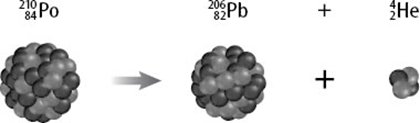
Nuclear Fission
Which group of elements on the periodic table has 4 valence electrons?
A. Group 4A
B. Group 5A
C. Group 6A
D. Group 7A
A. Group 4A
Which is an explanation of why solid copper wires are effective conductors of electricity?
A. electrons move easily within the wire
B. copper does not contains any protons
C. copper contains the same number of protons and neutrons
D. protons and electrons tend to cluster at opposite ends of the wire
A. electrons move easily within the wire
What is instantaneous Velocity?
The velocity of an object at a specific or exact moment in time.
The property of a material which describes how fast thermal energy transfers from one end of a material to the other end is called.
A. thermal essence
B. thermal equilibrium
C. thermal resonance
D. thermal conductivity
D. thermal conductivity
How does Iodine - 131 compare to the most common form of Iodine (Iodine - 126)?
A. iodine -131 has extra electrons
B. iodine -131 has extra neutrons
C. iodine -131 has extra protons and neutrons
D. iodine -131 has extra protons and electrons
B. iodine -131 has extra neutrons
Material that has a definite volume but no definite shape is a
A. Liquid
B. Solid
C. Gas
D. Plasma
A. Liquid
Which of the following is a chemical change?
A. Ice melting
B. Ice being carved
C. Water Boiling
D. Water separating into hydrogen and oxygen
D. Water separating into hydrogen and oxygen
How do you calculate Average Speed?
Give the formula
Speed = Distance divided by time
V = D / t
According to the periodic law, which element would most likely have similar properties to potassium?
A. Argon (Ar)
B. Chlorine (Cl)
C. Sodium (Na)
D. Calcium (Ca)
C. Sodium (Na)
In a tug of war, one team is pulling with a force of 100N to the left, the other team is pulling 80N to the right. What is the Net Force (magnitude and direction)?
A. 20 N to the left
B. 180 N to the left
C. 20 N to the right
D. 80 N to the right
A. 20 N to the left
A sailboat is moving at a constant velocity of 8 km/h eastward.

Which direction would you draw a vector for the friction force of the boat against the surface of the water?
A. to the East
B. to the West
C. water has no friction
D. upwards, away from the water
B. to the West
An ambulance siren sounds different as it approaches you then when it moves away from you. What term would you use to explain hos this happens?
A. Wave Refraction
B. Doppler Effect
C. Wave Diffraction
D. Wave Refraction
B. Doppler Effect
How is distance different from displacement?
Distance is the total amount an object has traveled. Always positive
Displacement is the amount away from the origin. Can be positive or negative
The element iron has seven naturally occurring isotopes. Which of the following describes the relationship of these isotopes?
- same mass, same atomic number
- different mass, same atomic number
- same mass, different atomic number
- different mass, different atomic number
B. different mass, same atomic number
Which of the following types of waves do not require a medium?
A. sound waves
B. seismic waves
C. ocean waves
D. electromagnetic waves
D. electromagnetic waves
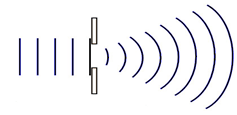
This diagram shows which of the following wave behaviors?
A. reflection
B. refraction
C. diffraction
D. absorption
C. diffraction
Which elements are least likely to react with metals?
A. nonmetals
B. other metals
C. noble gases
D. transition metals
C. noble gases
Which of the following objects has the greatest inertia?
A. a 10-kg mass traveling at 5.0 m/s
B. a 10-kg mass traveling at 10 m/s
C. a 15-kg mass traveling at 5.0 m/s
D. a 5.0-kg mass traveling at 15 m/s
B. a 10-kg mass traveling at 10 m/s
What is the weight on Earth of an object that has a mass of 5.00 kg?
A. 5 N
B. 50 N
C. 20 N
D. 200 N
B. 50 N
Which of the following elements are most likely to form an ionic bond?
A. Sodium bromo
B. Sodium bromine
C. Sodium bromide
D. Monosodium bromide
C. Sodium bromide
Which of the following equations is correctly balanced for the decomposition of hydrogen peroxide?
A. H2O2→2H2O+O2
B. 2H2O2→2H2O+O2
C. 2H2O2→2H2O+2O2
D. 2H2O2→3H2O+O2
B. 2H2O2→2H2O+O2
Which of the following supports and provides evidence for the big bang theory?
A. the presence of other galaxies
B. the motion of plants
C. the red shifting of starlight
D. the blue shifting of starlight
C. the red shifting of starlight
What is the acceleration of the object depicted in the graph?
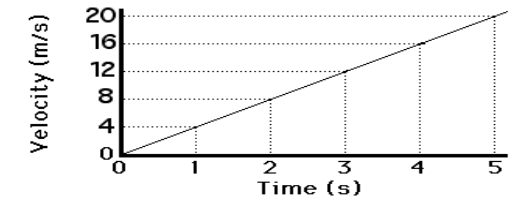
- 8 m/s2
- 4 m/s2
- 1 m/s2
- 0 m/s2
B. 4 m/s2