
How many bond pairs and lone pairs are present on the Phosphorous atom?
3 bond pairs
1 lone pair
Of the three bond types, which is the strongest?
Ionic Bond
What is the bond angles of trigonal planar-shaped molecule?
120o
What property makes H2O the universal solvent?
Hydrogen Bonds
What is the ideal gas equation?
PV=nRT
When predicting the structure of a molecule, what is used to find the stability of the proposed structures?
Formal charges
Which of the following is the correct definition for electronegativity?
a) Half the distance between the nuclei of two bonded atoms
b) The amount of energy required to remove an electron
c) the tendency of an atom to attract a shared pair of electrons towards itself
d) All of the above correctly explain electronegativity
Answer C
What molecular shape has a bond angle of 109.5o?
Tetrahedral
Does London Dispersion forces result in a permanent or temporary bond?
Temporary bond
What are the conditions of an ideal gas?
273 K and 1 atm
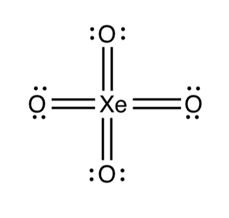 How is this an exception to the octet rule?
How is this an exception to the octet rule?
Xenon in this molecule possesses more than 8 electrons.
What elemental family has the highest electronegativity value?
Halogens
What electron repulsion is strongest within a molecule between lone-pairs or bond pairs?
Lone-pair to lone-pair repulsion
What are the two phases in chromatography?
Stationary phase and Mobile Phase
Hydrogen gas is collected over water. The total pressure of the mixture at 295 K is 785.2 torr. The vapor pressure of water at 295K is 19.8 torr. What is the pressure of the dry gas?
765.4 torr
If the electrons of a double bond are delocalized, the molecule has what?
Resonance
What electronegativity term is defined by "how much an atom attracts electrons"?
Electron Affinity
How many electron domains does a trigonal pyramid-shaped molecule have?
2 electron domains
If an SO2 molecule has a domain of three, with two bond pairs and one lone pair, does the molecule have a dipole moment?
Yes
If provided the total pressure of gases what else do you need to find the partial pressure of one of the gases?
The mole fraction of the gas you are trying to find the partial pressure of.
Draw the Lewis Dot Structure for the molecule H2O2

Carbon had an electronegativity value of 2.5. This is bonded with Oxygen, which has an electronegativity value 3.5. What type of bond is this?
Polar Covalent Bond
If a molecule has a domain of 5, with 2 bond pairs and 3 lone pairs, what is the molecular shape?
Linear
How many sigma bonds and how many pi bonds does N2 have?
1 sigma bond
2 pi bonds
If a 4 L container of a gas at 2 atm is added to a 6 L container of a gas at 4 atm, what is the atomic pressure of the gases if transfered to a 2 L container?
16 atm