Two elements with different numbers of these are considered isotopes
Neutrons
How many significant figures are in 100.029?
6
Which types of radioactive decay are occurring here?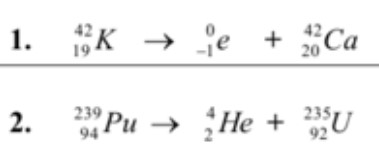
1. Beta decay
2. Alpha Decay
What is the percent composition of Carbon in 300 g of CO2? Round to the nearest whole percent
33%
What kind of elements can be generally found in the p block?
Determine the number of neutrons for the following isotopes
1. Potassium-39
2. Cobalt - 59
1. 20 Neutrons
2. 32 Neutrons
Determine the number of significant figures and the scientific notation of 1,000,280.
It has 7 sig figs and It's scientific notation is 1.00028 x 107
Predict the product of this reaction
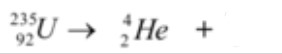
Thorium 239
What is the percent composition of hydrogen in 900 g of H2S?
67%
In group 15, which element... (1) is the heaviest (not livermorium), (2) is the fewest electrons, (3) is a metalloid and (4) is post-transition metal?
(2) Oxygen
(3) Tellurium
(4) Polonium
Determine the mass number for the following
1. Cerium with 82 neutrons
2. Iridium with 115 neutrons
3. Polonium with 125 neutrons
1. 140
2. 192
3. 209
Determine the number of significant figures and the scientific notation of 0.00238 and 10,020,371.
0.00238 | 3 sig figs, 2.38 x 10-2
1,020,371 | 7 sig figs, 1.0020371 x 108
Infer the reactant of this reaction

25%
Challenge: What elements have the following electron configurations... 1s22s22p63s1 & 1s22s22p63s23p64s23d9
Sodium & Copper
Which has the largest mass number?
Strontium with 49 neutrons
Beryllium with 5 neutrons
Curium with 10 neutrons?
Curium!
Strontium-87
Beryllium-9
Curium - 106!
Determine the number of significant figures and the scientific notation of 0.3918 , 100.283, and 120,019.0
0.3918 | 4 sig figs , 3.918 x 10-4
100.283 | 6 sig figs, 1.00283 x 103
120,019.0 | 7 sig fig, 1.200190 x 105
Determine the reactants of each nuclear equation! Write them using their chemical symbol, mass number, and proton number.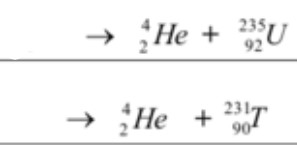
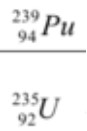
Nitrogen and oxygen are both in a tank. They are combined to create N2O5. What is the percent composition of Oxygen if 700 g of N2O5 is produced?
71%
Write the correct isotope notation for these isotopes. Ex: 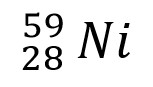
1. Cerium with 82 neutrons
2. Iridium with 115 neutrons
3. Polonium with 125 neutrons
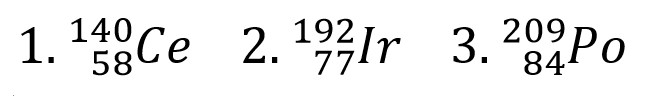
Determine the number of significant figures and the scientific notation of 1.3918 , 129.901 , 19.0, and 0.1212121
1.3918 | 5 sig figs, 1.3918 x 100
129.901 | 6 sig figs, 1.29901 x 102
19.0 | 3 sig figs, 1.9 x 101
0.1212121 | 7 sig figs, 1.212121 x 10-1
Predict the products of these nuclear reactions. 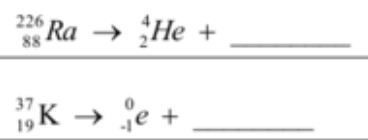
Equation 1 = Thorium 230
Equation 2 = Calcium 37
79%