John dalton improved on Democritus's previous ideas of the atom, what did he do that Democritus did not.
What is experimentation?
Write the abbreviated electron configuration for sodium
[Ne]3s2
Define Atom
What is the smallest object that retains the properties of an element?
Contains protons neutrons and electrons.

The name of this atomic model.
What is the Bowling ball model ?
This person discovered the electron
Who is JJ Thompson?
One similarity and one difference between the quantum mechanical model and the bohr model.
Both have energy levels associated with electrons, higher energy levels are located farther away from the nucleus of the atom
Both models allow electrons to jump energy levels and release a photon of light
Bohr model assigns electrons orbit in fixed line, where QMM is a probability cloud.
Quantum mechanical model is a mathematical approach.
Write the unabbreviated electron configuration for Bromine
What is
1s22s22p63s23p64s23d104p5
Define Isotope
What is two or more atoms that have the same atomic number (the same number of protons) but a different number of neutrons.
Ex: Carbon- 12 , Carbon-13, Carbon 14
The name of this atomic model.
This person is accredited for discovering the positively charged nucleus.
Who is Earnest Rutherford?
This person conducted the gold foil experiment
Who is Earnest Rutherford.
What is the electron configuration for Silver ?
What is
1s22s22p63s23p64s23d104p65s24d9
Define orbital and give the shapes of the main orbitals.
What is a mathematical function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus.
s- Sphere
P - Dumbell
d- Cloverleaf
The name of this atomic model

What is Planetary model,
or
Orbital model ?
This person created the first atomic theory
Who is John Dalton?
These TWO parts of John daltons theory were disproven.
What is the atom is indivisible and indestructible ?
What is the atoms of the same element are the same in mass and other properties?
Fill out this orbital diagram for oxygen

Define average atomic mass,
What is an average of all of the naturally occuring masses from their corresponding isotopes.
The name of this atomic model

What is Bohr's model?
This person discovered Neutrons
Who is James Chadwick ?
Why can you use line spectrum like the one below to identify an element.

Colors represent the wavelengths associated with the energy level of electrons in each element. Photons of light are emitted after electrons are excited and move energy levels.
Fill out this orbital diagram for Chlorine


!!!!!Daily double!!!!!
Hydrogen naturally occurs in three isotopes; Hydrogen-1, Hydrogen-2, Hydrogen-3.
Masses
Hydrogen-1: 1.0078 grams
Hydrogen-2: 2.0141 grams
Hydrogen-3: 3.0160 grams
If you had to guess the approximate percentages of these naturally occurring isotopes based off of hydrogens average atomic mass on the periodic table, What would they be ?
Hydrogen-1: 100% ( Technically, 99.98%)
Hydrogen-2: 0%
Hydrogen-3: 0%
The name of this atomic model
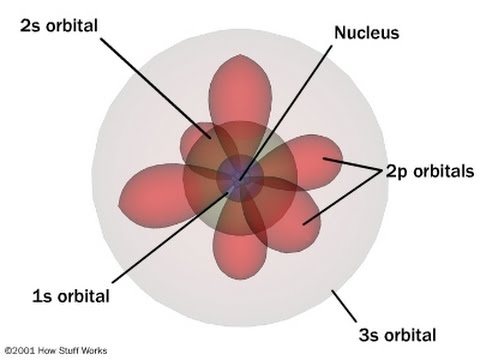
What is quantum mechanical model?
This scientist was the first to propose the atom as the smallest indivisible piece of matter.
Who is Dalton?