The following statement is true regarding liquids:
Small amounts of liquids can fill up a container.
If incorrect, fix the bolded term.
Statement is incorrect.
Small amounts of gas can fill up a container.
Carbon dioxide is classified as a(n)...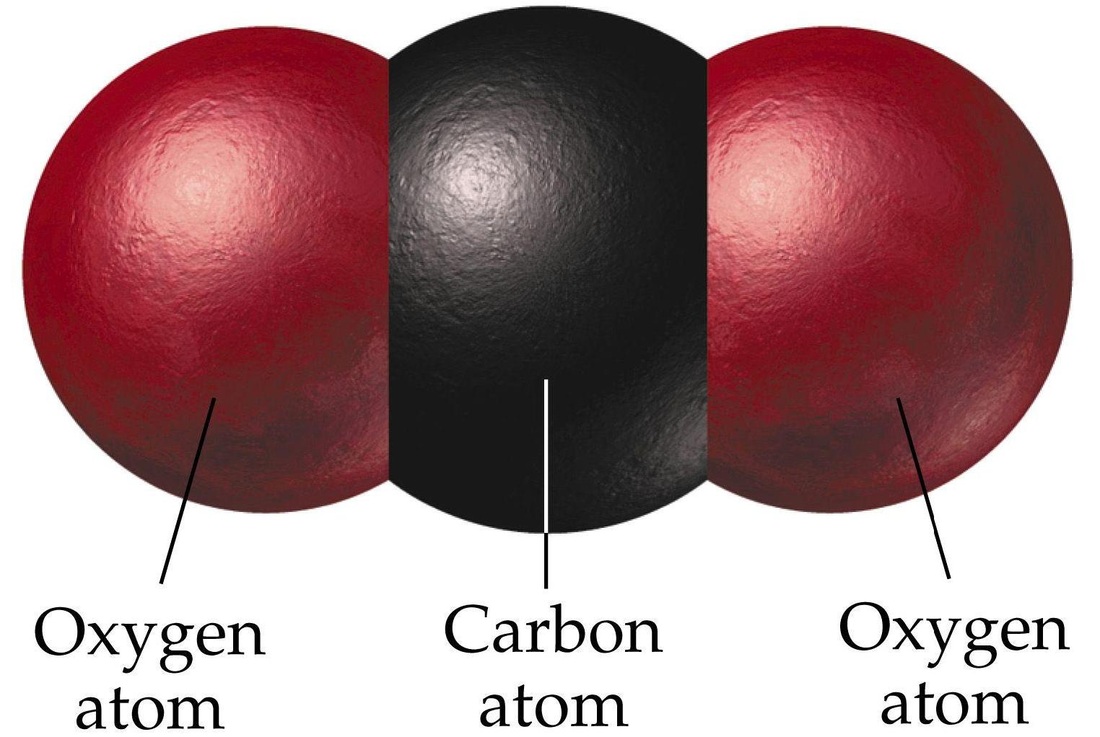
compound
(contains two different elements: carbon and oxygen)
Name 3 Physical Properties
Possible answers: Colour, Odour, Texture, Lustre, Clarity, Taste, State, Hardness, Malleability, Ductility, Melting Point, Boiling Point, Solubility, Viscosity, Density, Mass, Volume, Dimensions
Sublimation is the change from ______ to _____ and it is a _______ change.
Sublimation is the change from solid to gas and it is a physical change.
The density of water is...
approximately ~1g/cm3 or ~1 g/mL
Matter can be quantified in two ways. They are...
1._______?
2._______?
1. Mass
2. Volume
Solutions contains two parts: __________ and __________.
Solutions contains two parts: solutes and solvents.
Name three Chemical Properties
Possible Answers: Combustibility, Reactivity with Oxygen/Acid/Water, Sensitivity to Light, Flammability, Rusting, Corrosion
True or false: Melting and freezing happens at the same temperature
True!
Three liquids were poured into the same container. Liquid X has a density of 1.784 g/mL, Liquid Y has a density of 1.790 g/mL, and Liquid Z has a density of 1.789 g/mL. In order, from the bottom to top, will the liquids appear in once enough time has passed for the liquids to separate?
Top: Liquid X
Middle: Liquid Z
Bottom: Liquid Y

This image shows that...
Gas has mass.
List one example of each:
1. Heterogeneous mixture
2. Homogenous mixture
Class must agree that the two examples are valid examples of the mixtures classifications.
What is the difference between malleability and ductility?
Malleability: ability to be bent into different shapes
Ductility: can be pulled into long thin wires
Name two signs of chemical changes
Colour Change
Gas formation
Producing Electricity
Precipitation
The density of a liquid that has a mass of 4.0 g and a volume of 5.2 mL is...
0.77 g/mL
The process of dissolving shows that...
there is space between particles.
Name the three subcategories of Mechanical mixtures (heterogenous)
1. Ordinary mechanical mixture
2. Suspensions
3. Colloids
Name 3 physical properties
Possible answers:
Colour: copper/brown
Has lustre (shine)
Ductile
Solid
Frost forming on car windows during winter (gas to solid) is what change in state?
Deposition
The mass of an object with a density of 7.2 g/cm3 and a volume of 6.5 cm3 is...
46.8 g
There are 5 main ideas to the Particle theory of matter. They are....
1. All matter is made up of tiny particles.
2. Different substances are made up of different particles.
3. Particles are in constant motion.
4. Particles attract each other, and this force gets stronger as particles get closer.
5. Particles move faster when temperature increases.
Classify the following:
Heterogenous mixture: Suspension
Name the physical or chemical property:
Reactivity with water/acids/bases
The change in this is...
Precipitation
The volume of a gas that has a mass of 15.8 g and a density of 2.6 g/mL is...