This trend increases down Group 1
What is atomic radius?
What describes the electron behaviour in metallic bonding?
What are the criteria for a successful reaction?
1. What is particles must collide
2. What is energy great than activation energy
3. What correct geometry
What is a BL acid?
What is the effect of a catalyst?
What is decreases activation energy?
This element has the higher electronegativity?
What is fluorine?
Metallic and Ionic bonding both form this structure when forming bonds
What is a lattice?
Name at least three things that will speed up a chemical reaction?
What is higher temperature, smaller particle size (high surface area), higher pressure, catalyst, greater concentration?
What is the pH of a strong base with the concentration of 0.01?
What is 12
What is the effect of increasing temperature on a maxwell boltzman diagram?

What is increasing non metallic character?
What is increasing Zeff charge?
What is the shape (and bond angle) of methane (CH4)
What is tetrahedral (109.5)
What is missing on the x axis?
What is kinetic energy?
What is an artificial source of nitrous oxides and sulfur dioxide?
What is cars (transport)?
What is combustion of fossil fuels?
How do I calculate instantaneous rate at 60 sec
What is draw a tangent (delta Y/ Delta X)
Which elements would form the most polar bond?
What is fluorine and francium?
Name a substance with a resonance structure? Draw it's resonances?
What is benzene, ozone, carbonate?
What is the effect of a catalyst on a chemical reaction?
What is decreases activation energy?
What is provides an alternate reaction pathway?
What is the conjugate base of H2PO4-
What is HPO42-
Where does the reaction stop?

What is 4 and half minutes?
What is the trend in melting point in the halogens?
What is increasing (because of increasing dispersion forces)
What is likely identity of a substance that has a high melting point, low conductivity (when solid or molten).
Options-
Iron
Diamond
Sodium Chloride
What is diamond?
Label ΔH. Is this reaction exothermic or endothermic?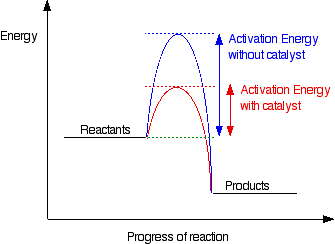
What is exothermic 
What is an amphoteric substance?
What is aluminium oxide (Al2O3)
Draw what would happen if the reaction was repeated with the same amount of substances except changing from chunks to powder?

What is A or B