This is the most simplest form of matter on earth.
What is the atom.
This property of matter can be viewed without technically changing the substances identity?
What is a physical property.
This term defines how much mass a substance has per the volume of it's container.
What is density.
This phase contains particles that are very dense and cannot moved from their fixed position.
What is the solid phase.
Which of the ideal gas laws demonstrates an inverse relationship between pressure and volume?
What is Boyle's Law.
This chemical property can be identified by an objects ability to burn.
What is flammability.
This formula can be used to calculate for density.
What is density=mass/volume.
This phase is represented by the particles in the image below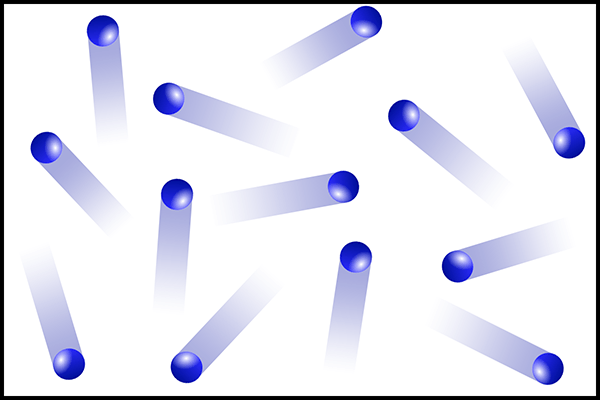
What is the gaseous phase
According to Charles' Law, as temperature goes up, volume goes...
What is up.
This cars ability to rust exemplifies irons ability to react with what periodic element to formulate rust?

What is oxygen.
Calculate the density of a 30 mL sample of ethyl alcohol with a mass of 23.71 g.
What is .79 g/mL
This phase change called sublimation changes a solid directly to what state?
What is a gaseous state.
Explain the type of change this piece of paper experienced to become an airplane and why it is that type of change.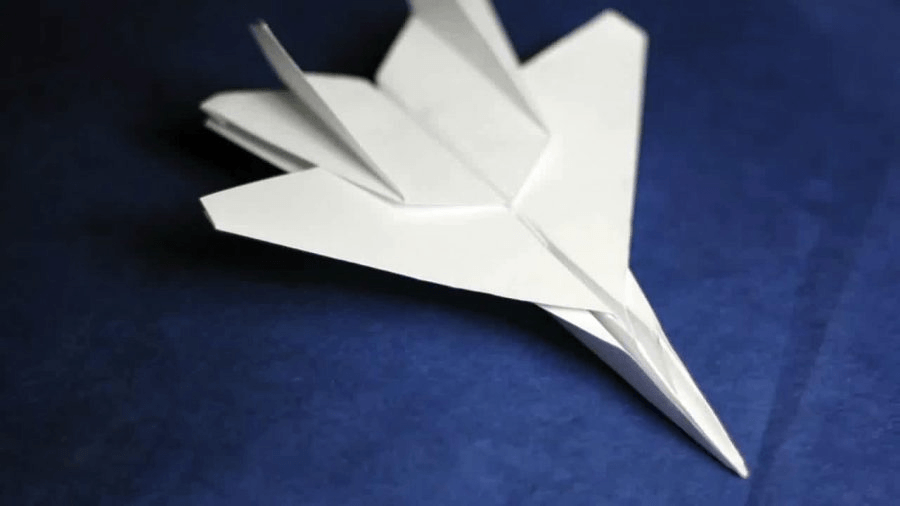
What is a physical change because the identity of the object did not change. It is still paper.
Find the mass of 250.0 mL of benzene. The density of benzene is 0.8765 g/mL.
What is 219.13 grams.
What is move more rapidly.
Identify at minimum 5 physical properties of matter and 2 chemical properties.
I'm not listing all these out!!!
28.5 g of iron shot is added to a graduated cylinder containing 45.50 mL of water. The water level rises to the 49.10 mL mark. From this information, calculate the density of iron.
What is 7.92 g/mL
This phase of matter can be identified as being very gas-like (having no definite shape or volume), but also has negatively charged electrons surrounding it.
What is the plasma state