What rule states that electrons will always occupy the lowest energy orbital first?
Aufbau Principle
What is the full electron configuration for Lithium (Li)?
1s2 2s1
This is used to describe the arrangement of electrons around a nucleus
What does EMR stand for?
Electromagnetic Radiation
What rule is this breaking?
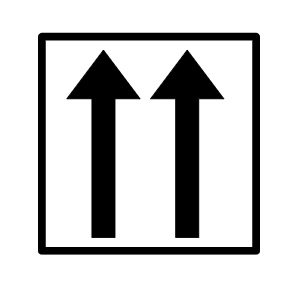
Pauli Exclusion Principle
What is the last occupies sublevel for Bromine (Br)?
4p5
An area of space around the nucleus where up to two electrons are likely to be found.
Orbital
What is the speed of light equation?
What rule is this breaking?
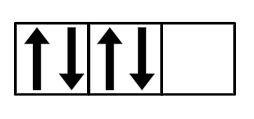
Hund's Rule
What is the last occupies sublevel for Osmium (Os)
5d6
A visual representation of electron configuration that shows relative energies.
Orbital Diagram
List the different types of radiation in order of decreasing energy.
Gamma Rays, X-Rays, UV, Visible Light, Infrared, Microwaves, Radio Waves
What element does this electron configuration represent?
Sulfur
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p2
Tin (Sn)
Orbitals that have exactly the same relative energy.
Sublevel
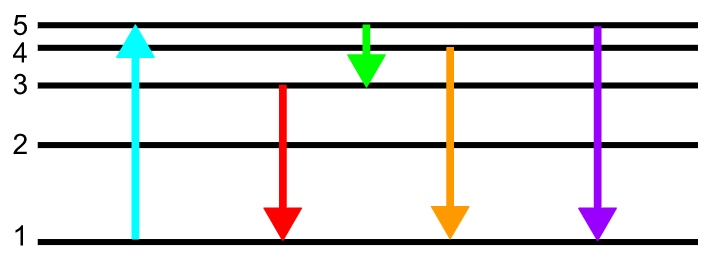
Which color arrow represents an electron releasing the least amount of energy?
Green
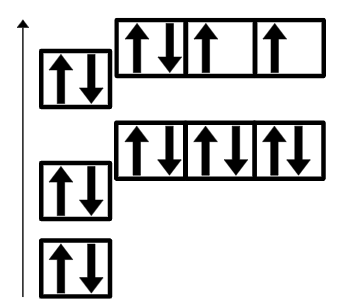
Draw the orbital diagram for Titanium (Ti)
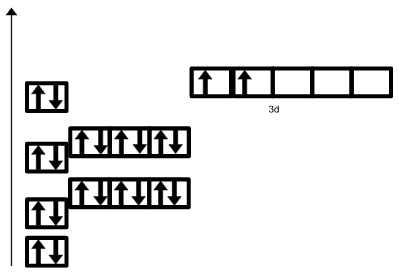
What is the noble gas notation for Bismuth (Bi)?
[Xe] 6s2 4f14 5d10 6p3
Ground State
If a EMR wave has a frequency of 1.56x1017Hz, what is the wavelength of this wave? (Answer in scientific notation)
1.92x10-9 m