What does STP stand for
Standard Temperature and Pressure
What are the three main particles that make up an atom and where are they located?
Protons and neutrons - in the nucleus
electrons -- in the electron cloud/shells
What happens to the number of energy levels as you move DOWN the periodic table?
They increase
What is the difference between a cation and an anion?
Cations are positively charged and anions are negatively charged
How many grams are in a mole?
the mass number on the periodic table
Balance the following equation: ___ N2 + ___ F2 --> ___ NF3
1 N2 + 3 F2 -> 2 NF3
If the enthalpy of a reaction is negative, is the reaction endothermic or exothermic?
Exothermic
What is the final answer with the correct number of significant figures?
2000 x 0.0046
9
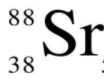 How many protons, neutrons, and electrons does this isotope of Strontium have?
How many protons, neutrons, and electrons does this isotope of Strontium have?
protons = 38
neutrons = 50
electrons = 38
How many valence electrons do elements in group 15 have and what kind of ion will they typically form?
5, anions
What is the molecular geometry of CH4?
Tetrahedral
What is the percent composition of Br in NaBr
77.7%
Show both the net ionic equations for the balanced reaction:
Na₃PO₄ (aq) + FeCl₃ (aq) → FePO₄ (s) + 3 NaCl (aq)
Net Ionic: PO₄³⁻ (aq) + Fe³⁺ (aq) → FePO₄ (s)
What law states that the entropy of the universe is always increasing for all natural processes.
The second law of thermodynamics.
How many significant figures?
7100
2
An electron has a wavelength of 720 nm. What is it's frequency? (Units and sig figs)
4.2 x 1014 Hz or 1/s
What is the main property responsible for increasing the atomic radius of elements as you move down the periodic table?
Additional energy levels further from the nucleus
What is a polar covalent bond?
When electrons are shared unequally because of a difference in electronegativity.
Molar Mass of Copper (1) Sulfate.
223.154 grams
Find the pH of a substance with an [H+] of 4.00 x 10-13 M
What is 12.4?
How much heat energy is required to raise the temperature of 55.0g of water from 25.0 degrees C to 28.6 degrees C if the specific heat of water is 4.18J/g degrees C?
q = 827.64 J
What do quantity do the coefficients in a chemical equation represent?
moles
If the mass number of Sodium is 23, how many neutrons does it have?
12
The electron configuration of copper?
1s2 2s2 2p6 3s2 3p6 4s2 3d9
Write the chemical formula for Tetraphosphorus Decaoxide
P4O10
DAILY DOUBLE
In this phase of matter particles can move quickly and freely, but not in all directions.
Liquid
N2H4 + 2H2O2--> N2 + 4H2O
What is the limiting reactant when .750mol N2H4 is mixed with .500 mol H2O2?
limiting reactant = H2O2
Calculate the Gibbs free energy change (G) for the following chemical reaction: ||| glutamate + NH3 --> glutamine + H2O ||| The reaction occurs at 25 °C, the change in heat = 500 kJ, and the change in entropy = 4000 J/°C.
400 kJ
What is the final answer with the correct number of significant figures?
6.88 + 970 + 2.19
980
I have three isotopes of carbon. Carbon 14 at 3.50% abundance, Carbon-12 at 85.15 % abundance, and Carbon - 13 at 11.35%. What is the average atomic mass of this sample of carbon?
12.18 amu
DAILY DOUBLE
1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p5
What element is this?
Iodine
Write the chemical formula for Copper (II) Nitrite
Cu(NO2)2
A 250 mL flask contains 3.4 g of neon gas at 45°C. Calculate the pressure of the neon gas inside the flask.
18 atm
DAILY DOUBLE
Cyanogen gas, C2N2, has been found in the gases of outer space. It can react with fluorine to form carbon tetrafluoride and nitrogen trifluoride.
C2N2 (g) + 7F2 (g) --> 2CF4 (g) + 2NF3 (g)
Find the volume of carbon tetrafluoride produced when 89.5g C2N2 is used.
77.1 L CF4
2S(s) + 3O2 (g) --> 2SO3 (g) with ΔH = 800 kJ/mol ||| 2SO3 (g) --> 2SO2 (g) + O2 (g) with ΔH = -200 kJ/mol ||| Based on this information, what is the ΔH for the following reaction: S(s) + O2 (g) --> SO2 (g)
300 kJ/mol
Write the empirical and molecular formulas of the following compounds:
A compound that is 52.2% Carbon, 17.4% Hydrogen and 30.4% Nitrogen and a molar mass of 92 grams per mole.
EF - C2H8N
MF- C4H16N2