If I have a mole of sodium chloride formula units, how many do I have?
What is 6.02 x 1023 formula units, Alex?
What is 5 moles, Ken?
Refer to the heating curve. At what letter(s) would a solid and liquid coexist and what is the name of that process?
What is letter b and melting?
List 3 things that would increase the pressure of a gas in a container and explain WHY the pressure is increased. Using the mathematical definition of pressure, explain why.
What is: 1) increase number of molecules in the container, 2) decrease the volume of the container and 3) increase the temperature of the container?
Since pressure is F/a, all three of these either increase the force of the molecules colliding with the container (1, 2) or decrease the area (3), thus increasing the pressure.
What is the electron configuration for the halogen in the 4th period? (and e- dot diagram)?
What is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p4?
Balance the following AND identify the reaction type.
FeCO3 + Al(OH)3 → Fe(OH)2 + Al2(CO3)3
What is
3FeCO3 + 2Al(OH)3 → 3Fe(OH)2 + Al2(CO3)3
and a double replacement reaction, Alex?
What type of radiation has the highest penetrating power (goes through the most things)?
gamma
What is 452,000 J?
Convert 900 Torr to atmospheres. Show your math.
900 Torr x 1 atm/760 Torr = 1.18 atm
What is the concentration of a solution that contains 0.25 moles of HBr is 500 mL of solution?
What is 0.5 M, Alex?
What are the missing coefficients for the chemicals in the reaction and why do we have to balance them?
Al2S3 + CaBr2 → CaS + AlBr3
What is because of the law of conversation of mass?
Al2S3 + 3CaBr2 → 3CaS + 2AlBr3
These are the two types of nuclear reactions:
1) When nuclei are joined together and
2) nuclei of atoms are bombarded with neutrons to form a daughter isotope and some form of radiation.
What is nuclear fussion and fission?
What does A, B and C represent? If this substance is at zero degrees and 6 atm, what could you do to get it to condense?
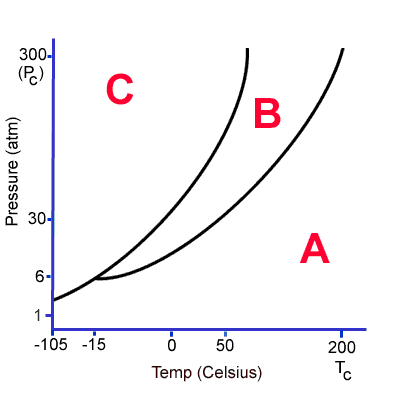
What is A-gas, B-liquid and C-solid.
What is lower the temperature or increase the pressure (keeping the other variable constant), Alex?
You bike a bag of chips from the base of a mountain 2000 feet in elevation. What happens to the bag and why, which law is this, and what is the relationship between the variables (show graph)?
The bag inflates because less air is pushing on it outside as the air mass thins (P down); Boyle's law, decrease air pressure, increase volume.
Consider the following reaction.
BaF2 (aq) + Li (s) → LiF + Ba
Will the reaction take place AND if so, what type of reaction takes place here?
What is yes and single replacement, Ken?
Label A, B and C AND determine if this is energy curve represents an endothermic or exothermic reaction.
A and B are the energy in the bonds of the reactants (chemical potential energy) and C is the energy in the bonds of the product.
Endothermic
All molecular motion stops at this temperature.
What is absolute zero or O Kelvin?
You open up a vacuum and nitrogen gas and neon gas diffuse in. This gas diffuses faster and this is the reason why.
What is neon because it's lighter and lighter chemical diffuse faster than heavier.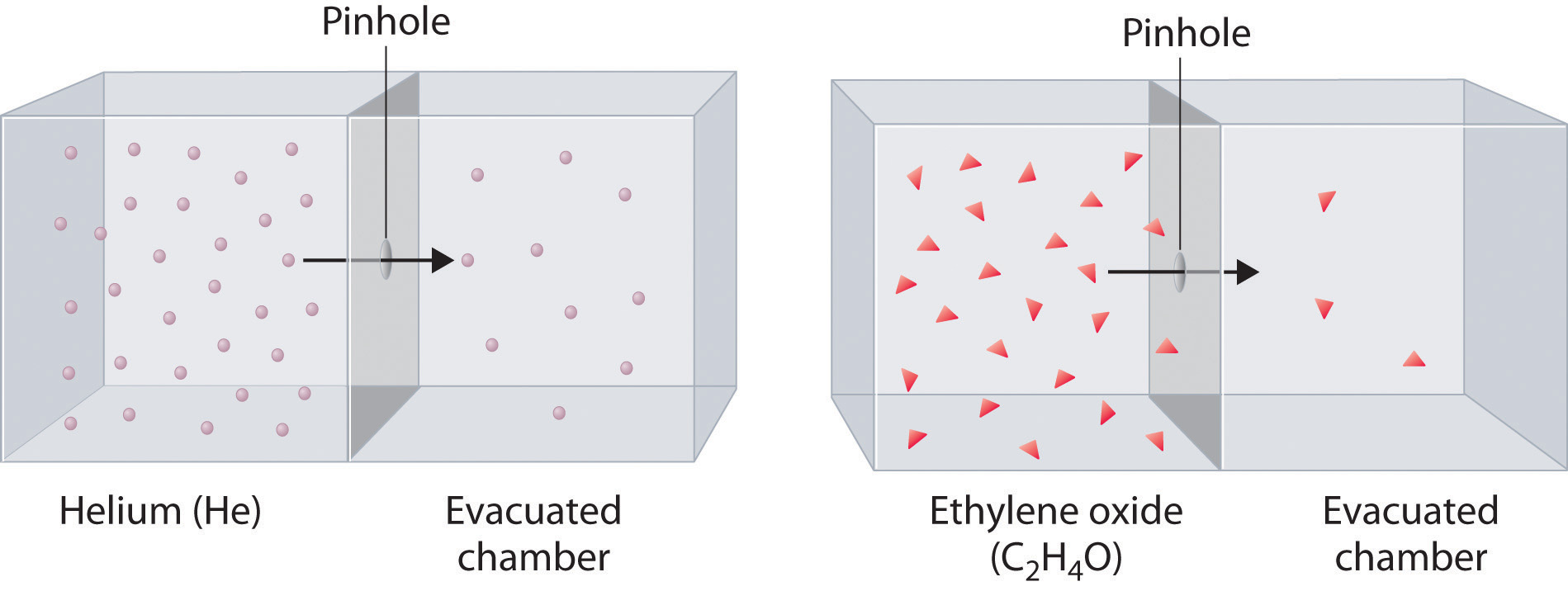
If 907 mL of carbon dioxide gas at 100 deg. C is transferred to a vessel at 43 deg. Celsius, what volume does the gas now occupy? Name the law. Show your math.
768 mL; Charles law
How much heat energy is required to boil 50 grams of water.
What is 113,000 J?
Q= mHv = 50g * 2260 J/g
Consider the following reaction. What three ways can we increase the number of collisions (and hence the reaction rate)?
BaF2(aq) + Li (s) ---→ LiF + Ba
What is
1) increase the concentration of the BaF2?
2) crush the solid lithium (to increase surface area)?
3) increase the temperature?
What are the products of Pu-239 alpha decay?
What is 125, 580 Joules of energy, Alex?
Nitrogen gas exerts a pressure of 0.9. atm at 25 deC. What temperature is required for the nitrogen gas to exert 1 atm (standard pressure) assuming constant volume? Name the law
What is 331.11 Kelvin?
Gay-Lussacs law.
The pressure reading of a tire is lower in the winter than in the summer. Whose law is this?
