Describe what happens in the following reaction in terms of bonds and energy:

What is bond are formed and energy is released (exothermic).
What type of bonds exist in CO2? [be specific]
What are polar covalent bonds.
Atmospheric pressure is caused by these.
These elements are found in the liquid state at STP.
What are Hg and Br.
The most probable location for an electron.
What is an orbital.
Draw the lewis dot diagram for Li2O.
What is 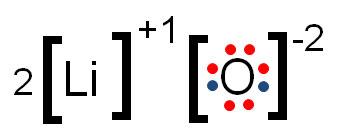
What type of bonding is depicted here?
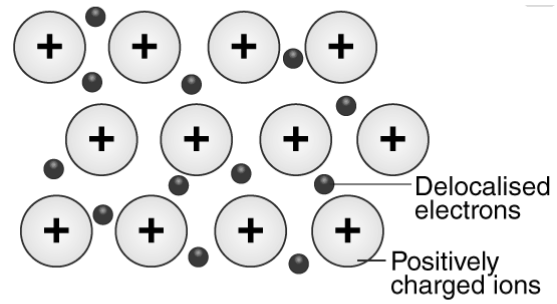
What are metallic bonds.
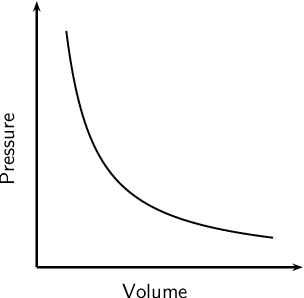
Draw a graph to show the relationship between pressure and volume at constant teperature.
What is
These have an incomplete "d" sub-level and form colorful solutions.
What are transition elements.
Dissolving in water is an example of what kind of change?
What is a physical change.
These are electrolytes.
What are ionic compounds.
These compounds are poor conductors, have extremely high melting and boiling points, and are not soluble in water.
What are Network solids.
Which sample would have the same number of molecules as 11.2L of He(g) at 273K and 202kPa?
1) 11.2L of N2(g) at 300K and 202kPa
2) 22.4L of Ne(g) at 546K and 404kPa
3) 11.2L of CH4(g) at 273K and 202kPa
4) 22.4L of H2(g) at 546K and 404kPa
What is 3) 11.2L of CH4(g) at 273K and 202kPa
Which sample of ethanol has particles with the highest average kinetic energy?
A) 10.0 mL of ethanol at 25°C
B) 10.0 mL of ethanol at 55°C
C) 100.0 mL of ethanol at 35°C
D)100.0 mL of ethanol at 45°C
What is B) 10.0 mL of ethanol at 55°C
O2 and O3 are examples of these.
What are allotropes.
Determine the molecular polarity of CF4.
What is nonpolar.
Determine the number of sigma and pi bonds in the following molecule:
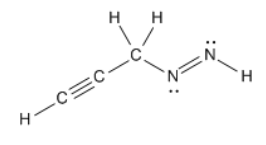
What are
1) 8 sigma
2) 3 pi
A cylinder is filled with 4.00 moles of nitrogen, 2.00 moles of hydrogen, and 6.00 moles of oxygen. If the gas mixture is at STP, what is the partial pressure of the hydrogen?
What is 0.167 atm or 16.9 kPa
Write and electron configuration for potassium in the excited state.
What is 2-8-7-2 or 1s22s22p63s23p54s2
Which phase of matter is being decribed here?
Definite volume
Takes shape of container
Free to move within sample
Slightly compressible
What is the shape of PCl5?
What is trigonal bipyramidal.
What is the hybridization for SF6?
What is sp3d2.
Calculate the relative rates of diffusion of NH3 and CO2.
What is NH3 will diffuse 1.6 times faster than CO2
What is the melting point of this substance at STP: 
What is 80oC.
Give the 4 quantum numbers for the 10th electron in sulfur.
What are
n = 2
l = 1
ml = -1
ms = -1/2