Symbols
Valence electrons
Electromagnetic Spectrum
Electron Orbitals
Misc
100
E
energy
100
Hydrogen (H)
1
100
Gamma Rays vs Xrays: Which has the larger frequency
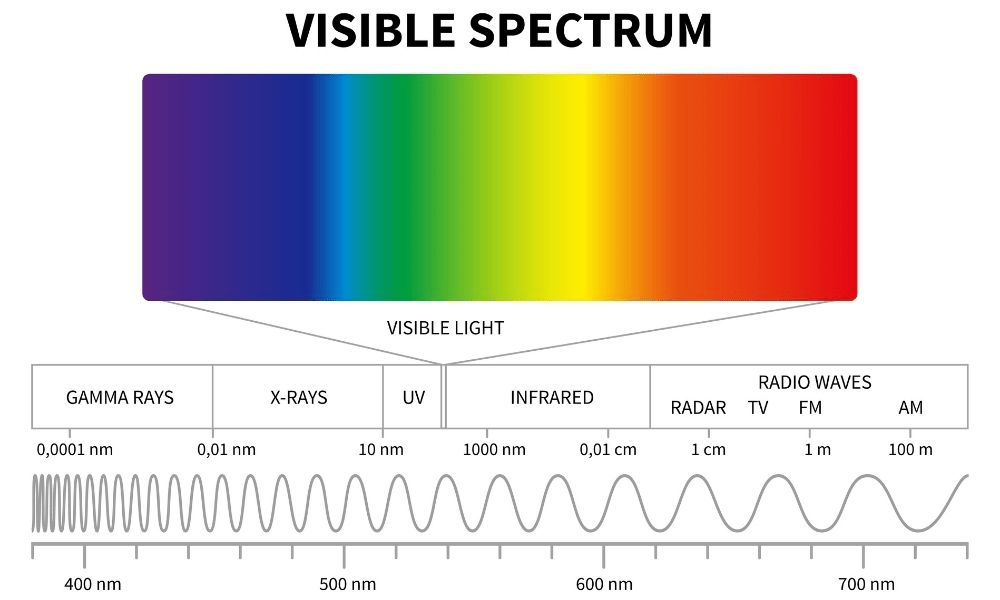
Gamma rays
100
1s2 2s2 2p6 3s2 3p4
S (sulfur)
100
Shape of d orbital
Clover

200
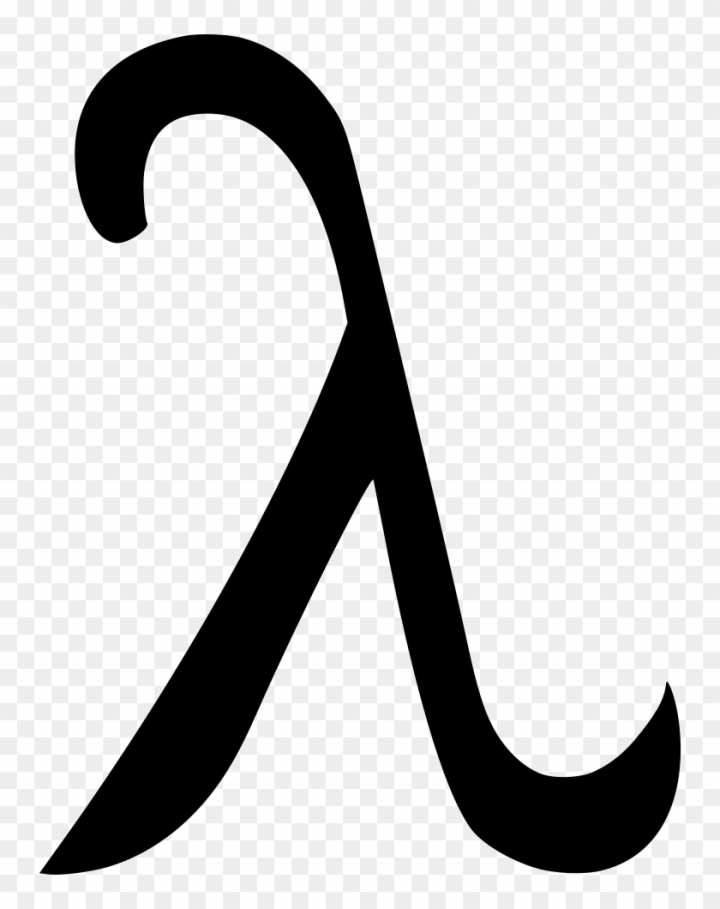
wavelength
200
Magnesium (Mg)
2
200
Violet vs Red: Which has the higher frequency
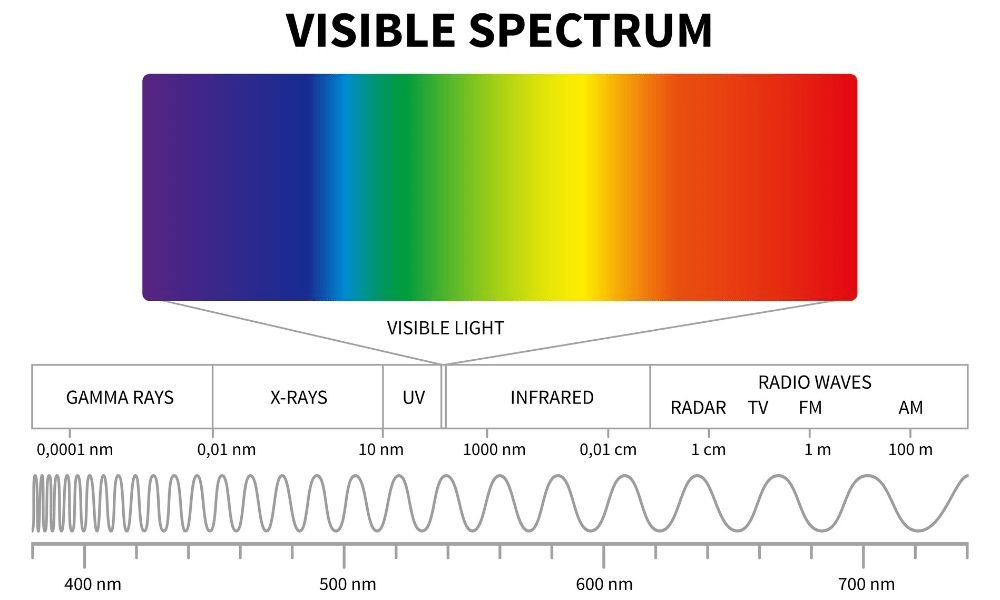
Violet
200
1s1
H (hydrogen)
200
As Frequency increases energy...
increases
300

Frequency
300
Fluorine (F)
7
300
Xray vs Infrared: Which has the longer wavelength
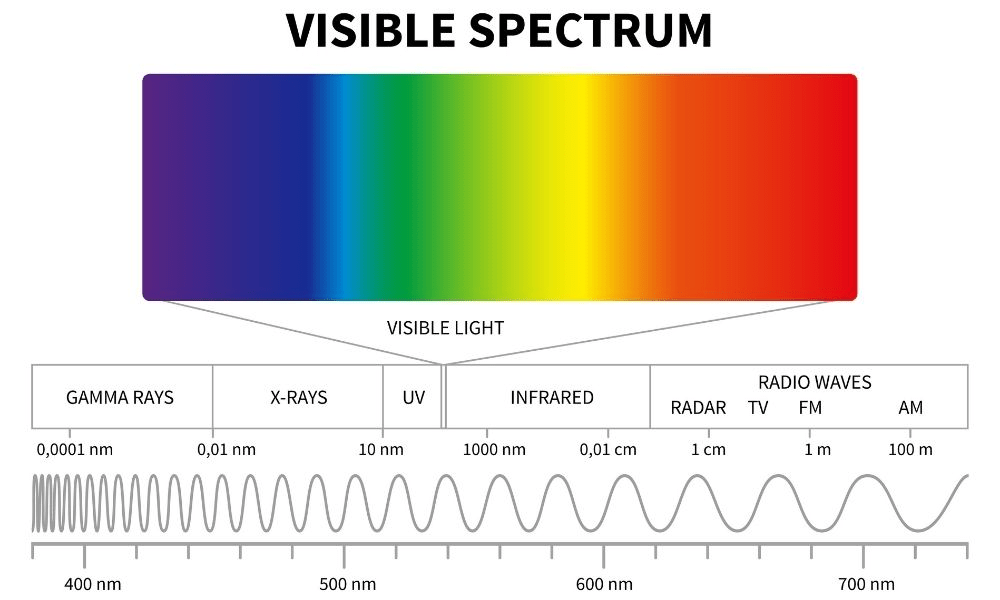
Infrared
300
1s22s22p5
F (fluorine)
300
As wavelength increases frequency...
decreases
400
c
Speed of light
400
Argon (Ar)
8
400
Green vs Yellow : Higher energy?
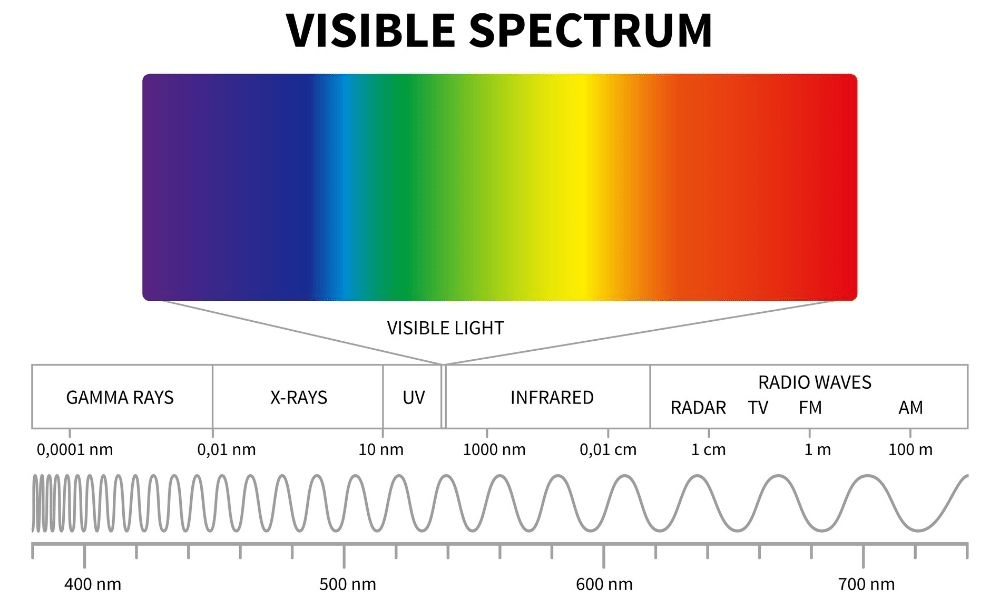
Green
400
1s2 2s2 2p6 3s2 3p6 4s2 3d8
Ni (Nickel)
400
Shape of the p orbital
Dumbell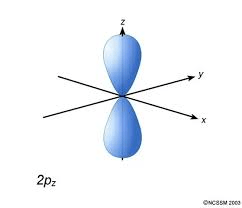
500
h
Planks Cosntant
500
Iodine (I)
7
500
FM vs AM: Which has the higher energy
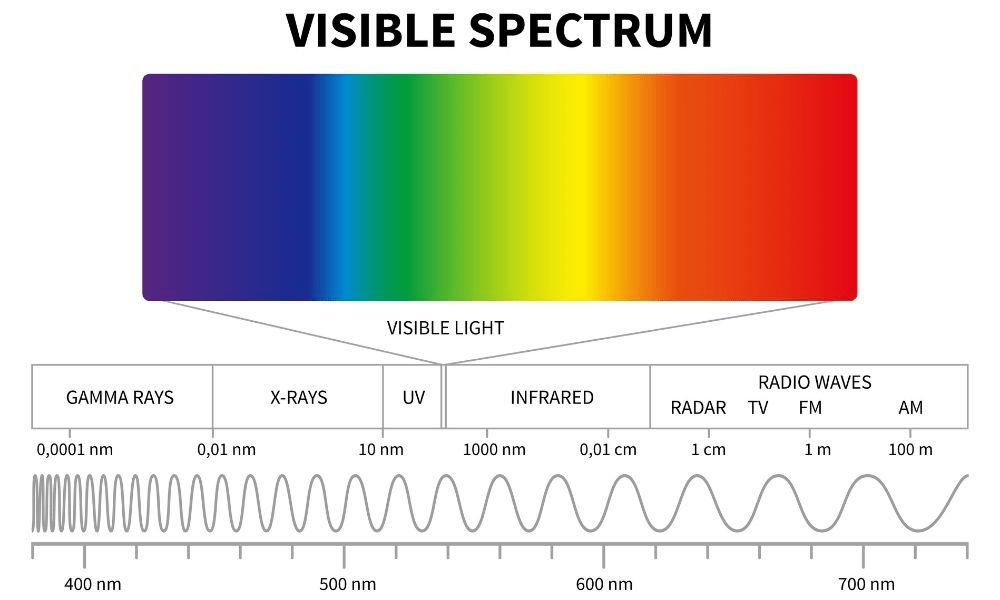
FM
500
1s2 2s2 2p6 3s2
Mg (magnesium)
500
As an electron moves from an excited state(higher energy) to a ground state (lower energy) what happens.
Energy is released