What is the oxidation state of Cl in Cl2
0
Which metal is more reactive, Ba or Mn?
Ba
What substance is oxidized in the following REDOX reaction?
Ca(s) + AlCl3(aq) --> Al(s) + CaCl2
Ca
What happens at the anode in a voltaic cell?
Oxidation / electrons lost
According to the electrochemical cell diagram,
Which substance would represent the anode?
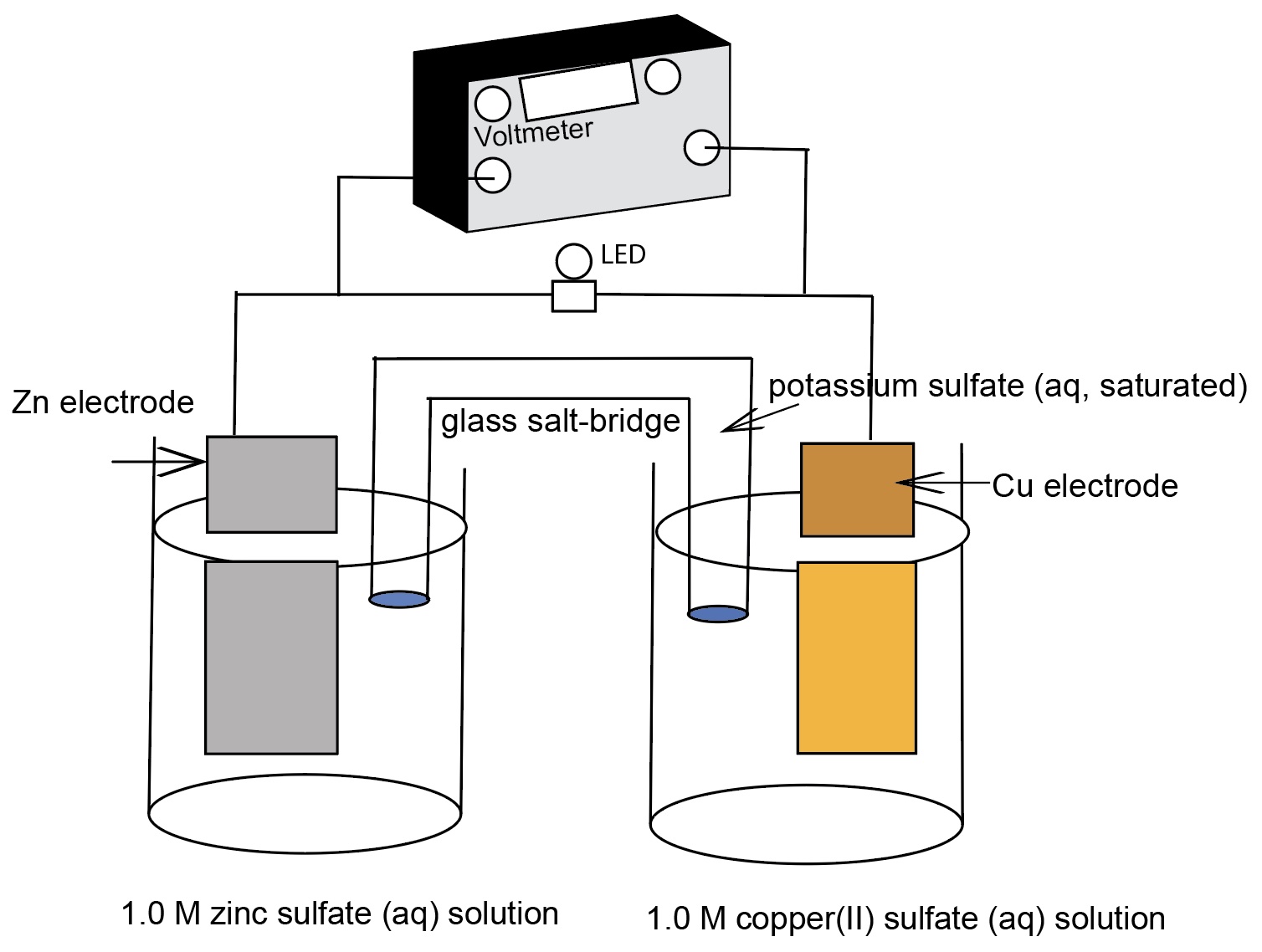
Zn
What is the oxidation state of Cr in Na2CrO4
+6
Which element is more reactive, F or I?
F
What substance is reduced in the following REDOX reaction?
2FeBr3(s) + 3F2(g) --> 2FeF3(s) + 3Br2(g)
F2
What is the charge of the cathode in an electrochemical cell?
positive
According to the electrochemical cell diagram,
Which electrode would gain mass as the cell progresses?
:max_bytes(150000):strip_icc()/saltbridge-56a12c293df78cf772681bf0.jpg)
Cu, Copper
What is the oxidation state of Fe in Fe(NO3)3
+3
Which element is more likely to be oxidized, Zn or Co?
Zn
How many electrons are transferred in the following unbalanced REDOX reaction?
Fe(NO3)2(aq) + Mn+2(aq) --> Mn(NO3)7 + Fe+3
5 electrons
Where are electrons transferred in an electrochemical cell?
Through the wire
According to the electrochemical cell diagram,
What would be the direction of flow of electrons?

From Mg to Ag
What is the oxidation state of Mn in KMnO4
+7
Which element is more likely to be reduced, Cl or Br?
Cl
When the following redox reaction is balanced, what is the coefficient in front of I-1?
Fe3+ + I-1 → Fe2+ + I2
2
Which electrode will negative ions flow in an electrochemical cell?
anode
According to the electrochemical cell diagram,
Would the pencil that is producing oxygen gas, O2, be the anode or the cathode?
Anode
What is the change of oxidation state for carbon, C, in the reaction CH4 + 2O2 --> 2H2O + CO2?
-4 to +4
Which element is more likely to be oxidized, Cu or F?
When the following redox reaction is balanced, what is the coefficient in front of NO2-?
N2O4(g) + Br-1 + OH-1 → NO2-1 + BrO3-1 + H2O
6
A voltaic cell has an anode, cathode, electrolyte solutions, wires and a voltmeter that records the voltage output as 0. Why is the most likely reason there no voltage?
Missing a salt bridge
What direction would EACH ion in solution flow according to the diagram?
Na solid on the cathode, Cl bubbles on the anode